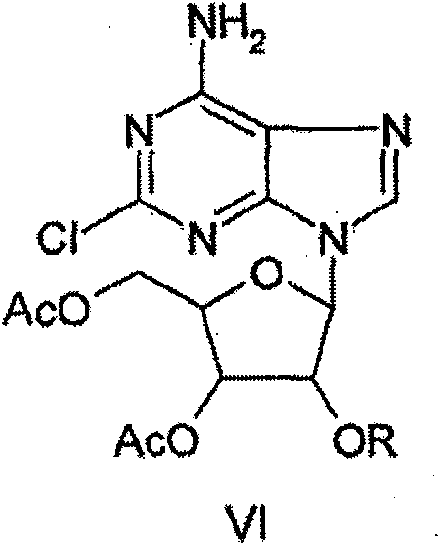
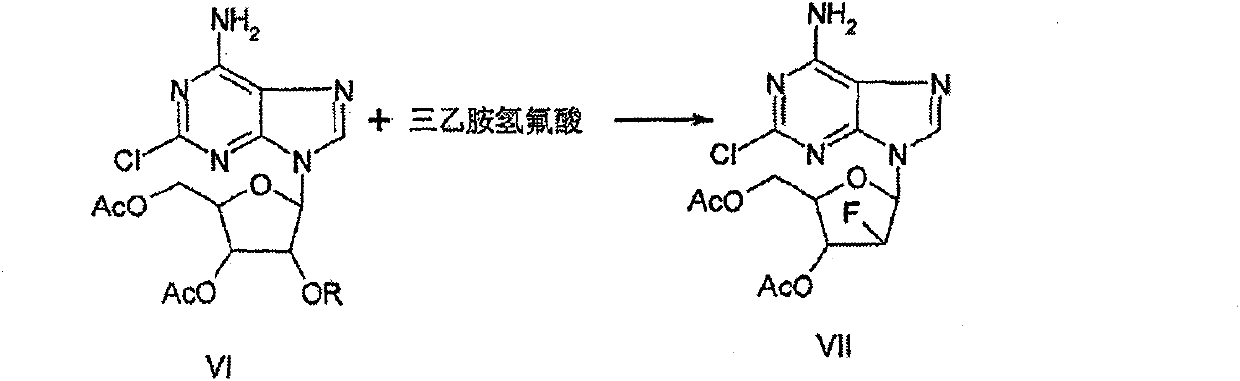
Synthesizing process of antineoplastic agent clofarabine
The technology of clofarabine and compound is applied in the field of new synthesis process of antitumor drug clofarabine, which can solve the problems of serious environmental pollution and high cost of starting materials, and achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of 2′, 3′, 5′-tri-O-acetylguanosine
[0049]
[0050] Add 100 grams of guanosine, 210 milliliters of acetic anhydride, 280 milliliters of DMF and 100 milliliters of pyridine into a three-neck flask, and heat up to 75-80° C. for 1.5 hours under stirring. After the reaction of the raw materials is complete, concentrate under reduced pressure to remove about one-third of the volume of the DMF and pyridine mixture, add 150ml of ether and 150ml of isopropanol, a white solid precipitates out of the solution, cool down to about 0°C, and filter to obtain 113 grams of solid. The yield is about 78%. 【Melting point: 225-232℃】 1 HNMR(DMSO) 2.05(s, 2'-Oac), 2.06(s, 3'-Oac), 2.12(s, 5'-Oac), 4.29(m, 4'-H), 4.38(m, 5' -H), 5.51(m, 3'-H), 5.81(t, 2'-H), 5.81(d, 1'-H), 6.56(s, 2-NH2), 7.94(s, 8-H )
Embodiment 2
[0051] Example 2: Preparation of 6-chloro-2', 3', 5'-tri-O-acetylguanosine
[0052]
[0053]
[0054]Add 113 grams of 2′, 3′, 5′-tri-O-acetylguanosine, 125 grams of benzyltriethylammonium chloride, 35ml of N, N-dimethylaniline, and 600ml of acetonitrile in a 1-liter three-necked flask , 150ml of phosphorus oxychloride, heat up to 77-83°C under mechanical stirring, reflux for 30 minutes, then cool to about 50°C and concentrate under reduced pressure to a small volume, add the residue to ice water, use 1L, 500ml, 500ml of Extract with dichloromethane, wash the dichloromethane phase with 5% sodium bicarbonate solution to neutrality, dry over anhydrous sodium sulfate, filter, concentrate dichloromethane under reduced pressure to a small volume, add 600ml of isopropanol to separate out a large amount of solids, A light yellow solid was obtained by filtration, and vacuum-dried to obtain 90 g of 6-chlorotriacetylguanosine with a yield of about 76%. 【Melting point: 143-146℃】 1...
Embodiment 3
[0055] Example 3: Preparation of 2,6-dichloro-2', 3', 5'-tri-O-acetyladenosine
[0056]
[0057] Add 90 grams of 6-chloro-2', 3', 5'-tri-O-acetylguanosine and 1.4 liters of dichloromethane into a 2-liter three-necked flask, stir and dissolve, then add 48 grams of antimony trichloride, and wait After the antimony trichloride was dissolved, tert-butyl nitrite was added dropwise, and the solution became cloudy with solid precipitation after the dropwise addition. After tert-butyl nitrite was dripped, reacted for 30 minutes. TLC showed that the raw material had reacted completely. Concentrate under reduced pressure to a small volume to take away tert-butyl nitrite. Add 1.5 L of dichloromethane again, filter to remove salt, dichloromethane phase Wash twice with 5% sodium bicarbonate, then wash twice with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure and add 200ml of ethanol to continue concentrating until solids...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap