Synthesis of medicinal biodegradable poly(epsilon-caprolactone) and application method thereof
A synthesis method and technology of caprolactone, applied in the field of polymer chemistry, can solve the problems of cumbersome process, harsh storage conditions, easy moisture absorption of butyl titanate, etc., and achieve improved reaction efficiency, mild and controllable process, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Bulk Polymerization of ε-Caprolactone
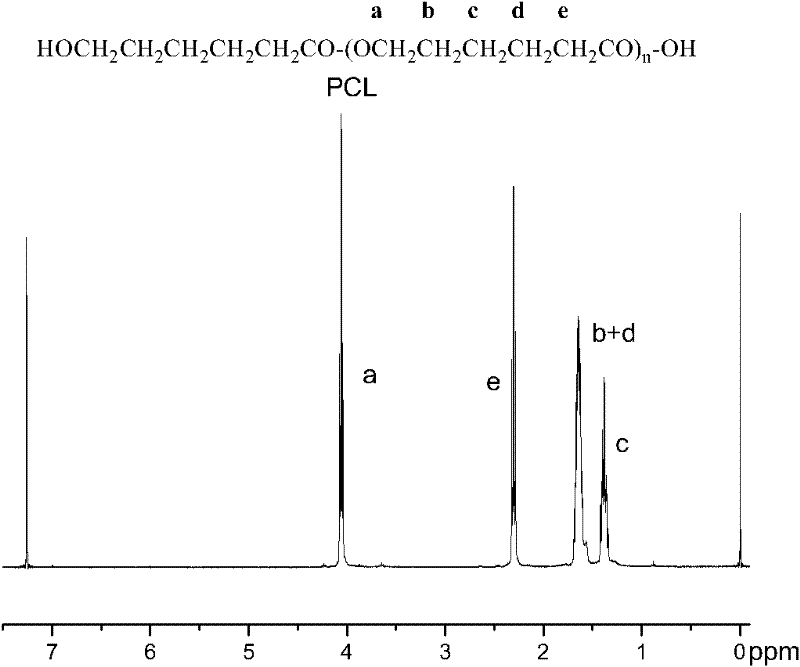
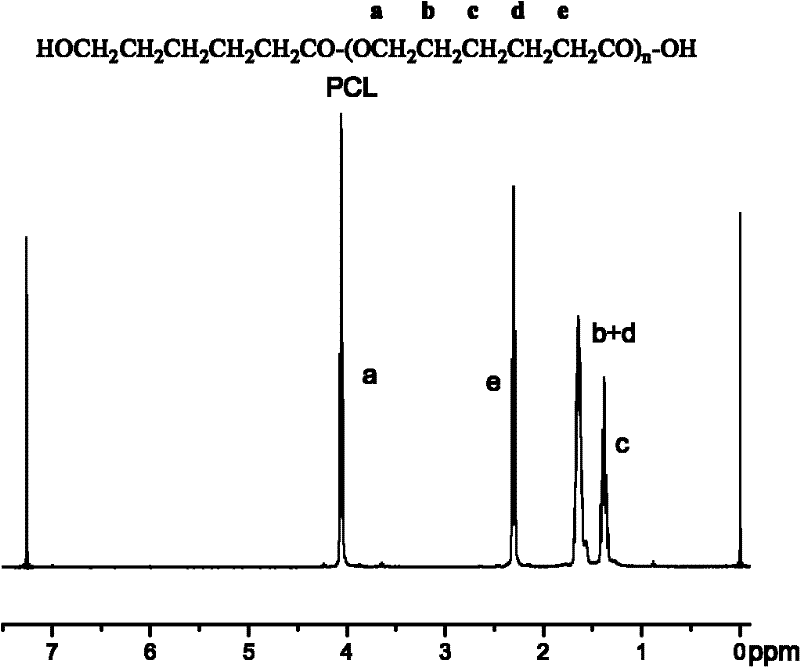
[0025] Mix 5g of ε-caprolactone with 0.05g of Novozym 435 (the mass ratio of ε-caprolactone is 1%), put it into a clean and dry reaction bottle, exchange it with nitrogen three times, and then depressurize to 2mm Hg pressure , shaking reaction at 60°C for 2h, adding 10ml of dichloromethane to the reactant to terminate the reaction, filtering to remove the enzyme, the filtrate was concentrated in vacuo, and the concentrated solution was subjected to proton nuclear magnetic resonance ( 1 H NMR) determined that the monomer conversion rate was 90.2%. The residual concentrate was poured into 5 times the volume of petroleum ether to precipitate, and the precipitate was collected after filtration and dried in vacuo for 48 hours. The product was determined by gel permeation chromatography (GPC) to have a number average molecular weight of 7000 g / mol and a polydispersity of 1.3.
Embodiment 2
[0027] Solution Polymerization of ε-Caprolactone
[0028] Mix 5g ε-caprolactone with 0.5g Novozym 435 (the mass ratio of ε-caprolactone is 10%), put it into a clean and dry reaction bottle, ventilate it with nitrogen three times, and add it with a syringe after sealing. -The volume ratio of caprolactone is 2.0:1 toluene, stirred and reacted at 80°C for 7h, added 10ml of dichloromethane to terminate the reaction, filtered to remove the enzyme, the filtrate was concentrated in vacuo, and the concentrated filtrate was subjected to nuclear magnetic resonance spectroscopy ( 1 H NMR) determined that the monomer conversion rate was 95.4%. The residual concentrate was poured into 5 times the volume of petroleum ether to precipitate, and the precipitate was collected after filtration and dried in vacuo for 48 hours. The product was determined by gel permeation chromatography (GPC), with a number average molecular weight of 42000 g / mol and a polydispersity of 1.2.
[0029] Such as fi...
Embodiment 3
[0032] Solution Polymerization of ε-Caprolactone
[0033] Mix 5g of ε-caprolactone with 1g of Novozym 435 (the mass ratio of ε-caprolactone is 20%), put it into a clean and dry reaction bottle, ventilate it with nitrogen three times, and add ε-caprolactone with a syringe after sealing. The volume ratio of the lactone is 5.0:1 toluene, stirred and reacted at 80°C for 10h, added 20ml of dichloromethane to terminate the reaction, filtered to remove the enzyme, the filtrate was concentrated in vacuo, and the concentrated filtrate was subjected to hydrogen nuclear magnetic resonance spectroscopy ( 1 H NMR) determined that the monomer conversion rate was 98.3%. The residual concentrate was poured into 5 times the volume of ethanol to precipitate, and the precipitate was collected after filtration and dried in vacuum for 48 hours. The product was determined by gel permeation chromatography (GPC) to have a number average molecular weight of 57000 g / mol and a polydispersity of 1.4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com