Treatment of pulmonary disorders with aerosolized medicaments such as vancomycin
A vancomycin, aerosolization technology, which can be used in therapeutic nebulizers, drug combinations, drug delivery, etc., and can solve problems such as harmful health effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
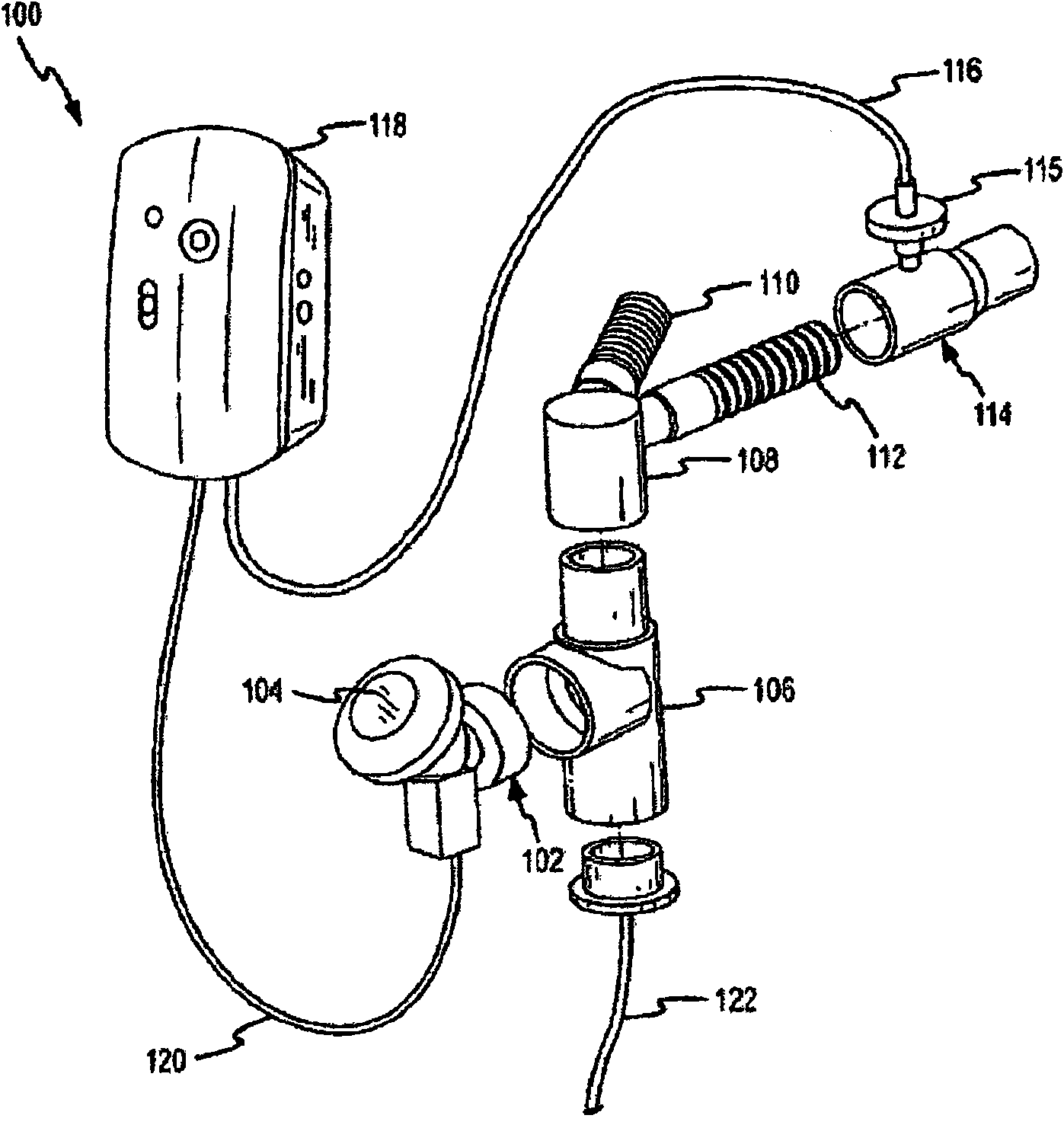
[0097] As noted above, conventional nebulizer-ventilator systems have low drug delivery efficiencies (eg, less than 20%). Embodiments of the invention include methods and systems that increase delivery efficiency to, for example, at least 25%, or at least 30%, or at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, or more. The increased efficiency in delivering aerosolized drugs may be due in part to one or more features that may be applied in embodiments of the invention. These features include synchronizing the generation of aerosol with the inhalation phase of the ventilator cycle (eg, staged delivery). These features may also include supplying air (eg, "air flow") following the generation of the aerosol, which can clean the endotracheal tube and reduce the amount of drug exhaled by the patient. These features may further include a hub connecting the aerosol generating unit directly to an endotracheal tube connected to the patient. Other features include...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com