High-barrier medical film
A high-barrier, thin-film technology, used in pharmaceutical containers, medical packaging, special packaging, etc., can solve the problems of poor reproductive system development, environmental impact of dioxins, poor barrier properties, etc., to avoid particles and other pollution, The effect of low water vapor transmission and good barrier properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] Below in conjunction with specific embodiment, further illustrate the present invention.
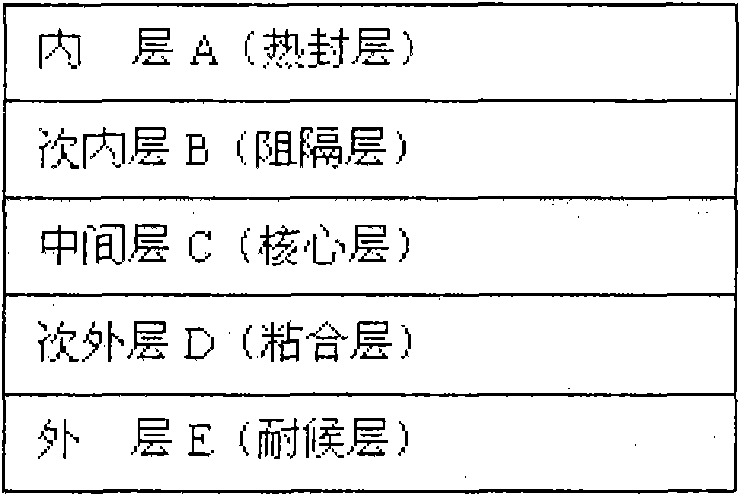
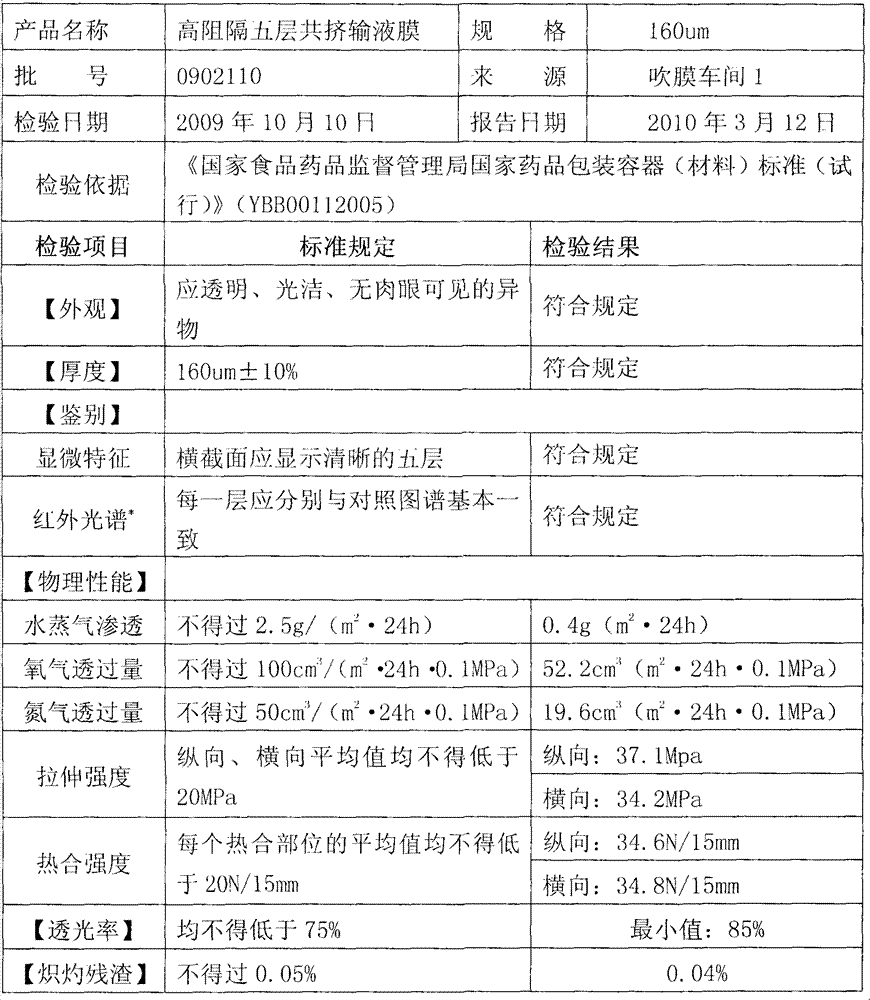
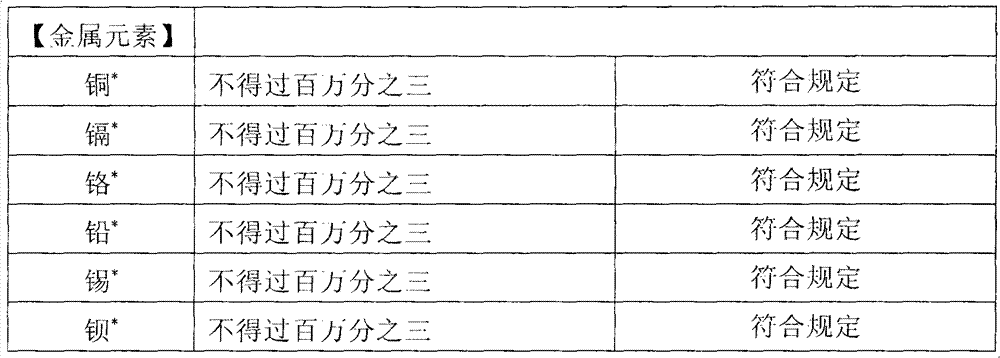
[0032] Such as figure 1 Shown is the structure distribution of each layer of the high-barrier five-layer co-extrusion infusion membrane of the present invention. Depend on figure 1 It can be seen that the high-barrier five-layer co-extrusion infusion film of the present invention has a five-layer structure in terms of structural composition, and each layer from the first layer to the fifth layer is respectively the inner layer A, the second inner layer B, the middle layer C, and the second outer layer. D and the outer layer E.
[0033] A mixed resin composed of high-melting metallocene polypropylene and ethylene-octene copolymer (EOC) is selected as the first inner layer A, that is, the heat-sealing layer, and the amount of EOC added to the mixture is 10% to 30%. The melt index of the blend is 2.0-8.0g / 10min, and the density is 0.88-0.91g / cm 3 ; Use cycloolefin copolymer (COC)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com