Method for producing tetrahydrofuran
A technology of tetrahydrofuran and hydrogen esters, which is applied in the field of tetrahydrofuran production, can solve the problems of catalyst deactivation, low hourly space velocity of ester liquid, and increased selectivity of by-products, and achieve high catalytic activity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
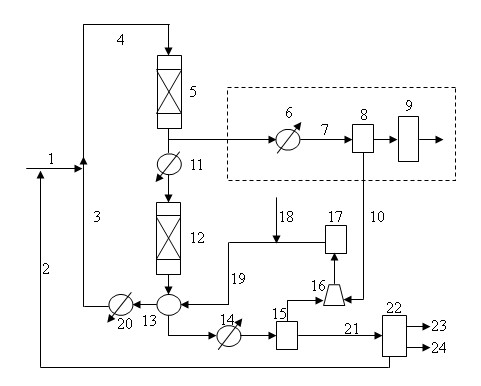
[0020] according to the invention figure 1 The technological process of this method uses different catalysts and cooperates with suitable technological conditions to carry out hydrogenation reaction with dimethyl maleate as a raw material. The specific conditions and reaction results are shown in Table 1, Table 2 and Table 3.
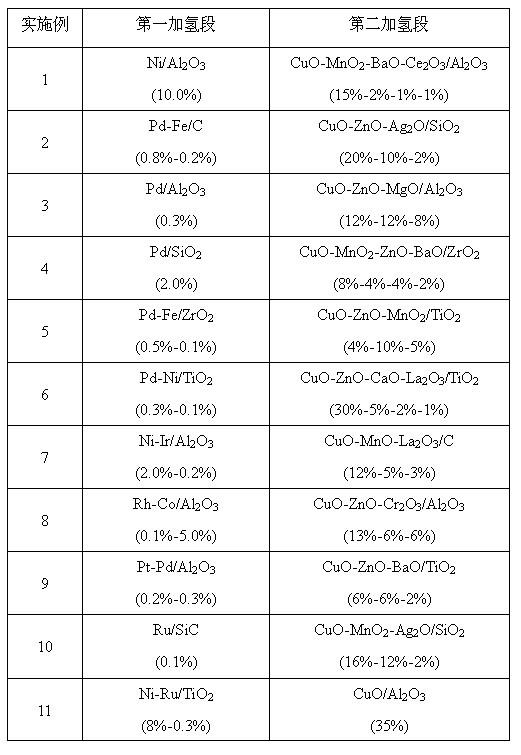
[0021] Table 1 Catalysts of Examples 1 to 11
[0022]
[0023]
[0024] .
Embodiment 12
[0026] Adopt the catalyzer of embodiment 3, dimethyl maleate is hydrogenated to produce tetrahydrofuran reaction and investigate, through 1500 hours of life test, catalyst activity and stability all maintain at relatively high level, and catalyst bed temperature rise is little and The n-butanol by-product is effectively controlled.
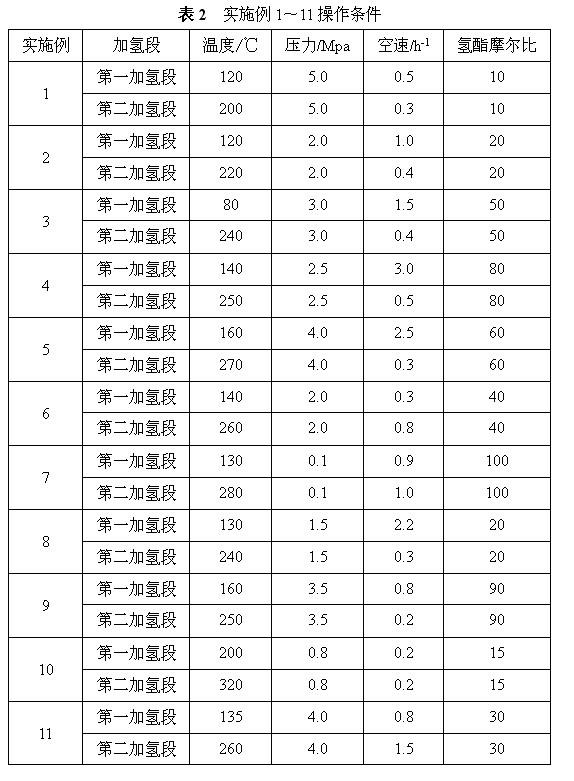
[0027]
[0028] Note: (1) Dimethyl maleate is completely converted into dimethyl succinate, and the conversion rate in the table refers to the conversion rate of dimethyl succinate; (2) The ester space velocity is 0.38g / (ml .h), the hydrogen ester molar ratio is 40, and the pressure is 3.0MPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com