Amoxicillin sulbactam pivoxil capsule and preparation method thereof
A technology of amoxicillin-sulbactam pivoxil and sulbactam pivoxil, which is applied in the field of pharmaceutical preparations, can solve the problems of large filling volume, poor patient compliance, poor dissolution effect of amoxicillin-sulbactam pivoxil capsules, etc. problem, to achieve the effect of simple preparation method, convenient taking, and enhanced compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Amoxicillin-sulbactam pivoxil capsule Specification: 250mg
[0029] (1) Weigh 3 kg of amoxicillin, 3.866 kg of sulbactam pivoxil, and 34 g of sodium lauryl sulfate.
[0030] Croscarmellose sodium 136g, magnesium stearate 49g.
[0031] The sulbactam pivoxil raw material, magnesium stearate, and sodium lauryl sulfate were passed through an 80-mesh sieve respectively and weighed, and amoxicillin, sulbactam pivoxil, and 1 / 2 amount of cross-linked carboxymethyl fiber Sodium plain is placed in a mixer, stirred and mixed, the mixed material is extruded in an extruder, and crushed to obtain dry granules, and the prepared dry granules are mixed with the remaining croscarmellose sodium, ten Dialkyl sodium sulfate and magnesium stearate are placed in a mixer and mixed to obtain a mixed powder. The mixed powder is inspected and filled into No. 1 capsule after passing the test. The filling amount is 0.345g / grain, and the drug content is 36.5% (calculated as amoxicillin.) The fill...
Embodiment 2
[0033] Amoxicillin-sulbactam pivoxil capsules Specification: 625mg
[0034] (1) Weigh 12kg of amoxicillin, 3.866kg of sulbactam pivoxil, and 79g of sodium lauryl sulfate.
[0035] Croscarmellose sodium 316g, magnesium stearate 111g.
[0036] The sulbactam pivoxil raw material, magnesium stearate, and sodium lauryl sulfate were passed through an 80-mesh sieve respectively and weighed, and amoxicillin, sulbactam pivoxil, and 1 / 2 amount of cross-linked carboxymethyl fiber Put plain sodium in a mixer to mix and stir to make it fully mixed, put the mixed material in an extruder to squeeze and crush to obtain dry granules, and mix the prepared dry granules with the remaining croscarmellose sodium , Sodium Lauryl Sulfate, and Magnesium Stearate are mixed in a mixer to obtain mixed powder. The mixed powder is inspected and filled into No. 0 capsules after passing the test. The filling amount is 0.77g, and the drug content is 65% (calculated as amoxicillin.) The filled capsules are ...
Embodiment 3
[0047] Stability study
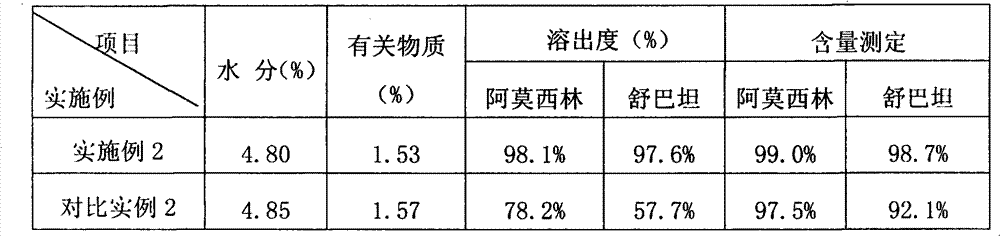
[0048] Objects of investigation: the product manufactured in Example 1 of the present invention and the product manufactured in Comparative Example 1.
[0049] Inspection method: Accelerated test is carried out under the conditions of relative humidity of 75%±5% and temperature of 40°C±2°C.
[0050] Inspection project: According to the "Standard YBHO1472007 of the State Food and Drug Administration", the product's properties, related substances, content, and dissolution rate were inspected.
[0051]The results are detailed in Table 1 and Table 2:
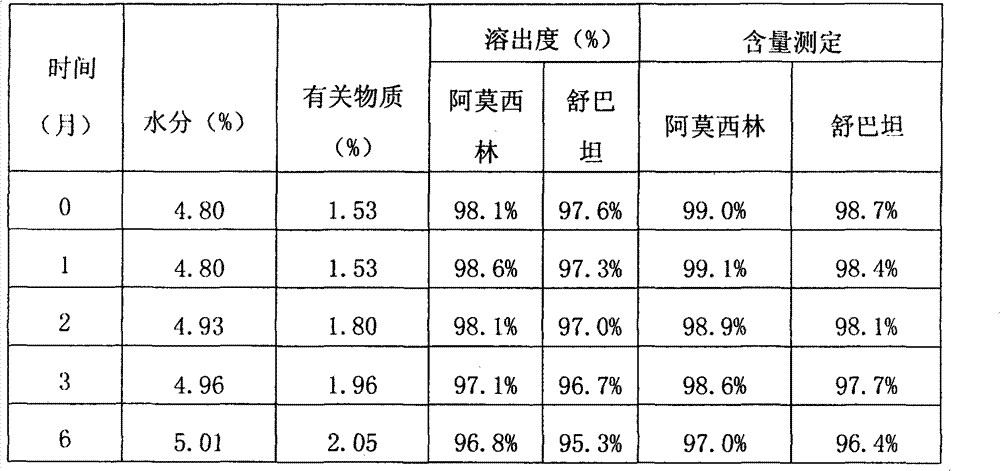
[0052] Table 1: Relevant data of the product made in embodiment 1
[0053]
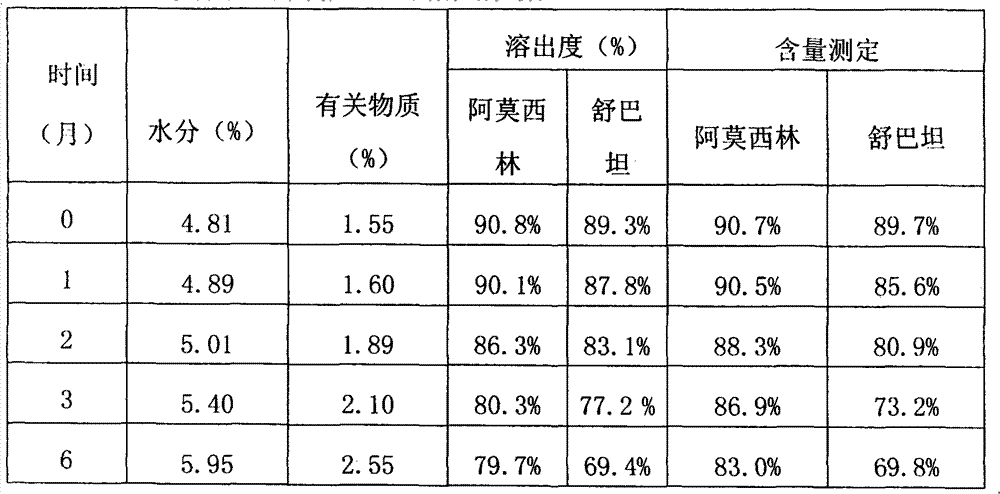
[0054] Table 2: Relevant data of the product made in comparative example 1
[0055]
[0056] As can be seen from the data comparison of Table 1 and Table 2, in the accelerated test, after placing 2 months according to the product manufactured by wet method, its related substances are obviously higher than the product manufa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com