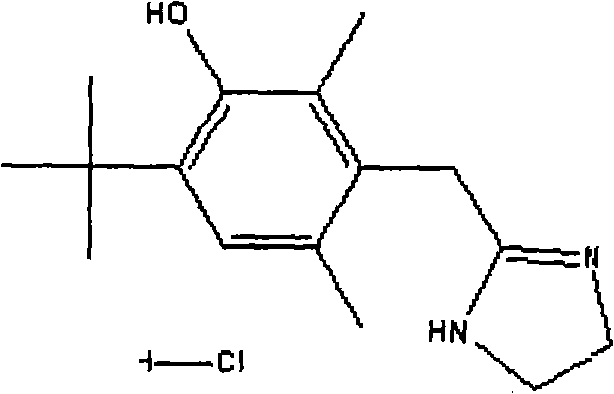
Enhancing photostabilization of oxymetazoline
A technology of oxymetazoline and composition, applied in the field of light-stable composition, capable of solving problems such as reducing exposure level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] combination
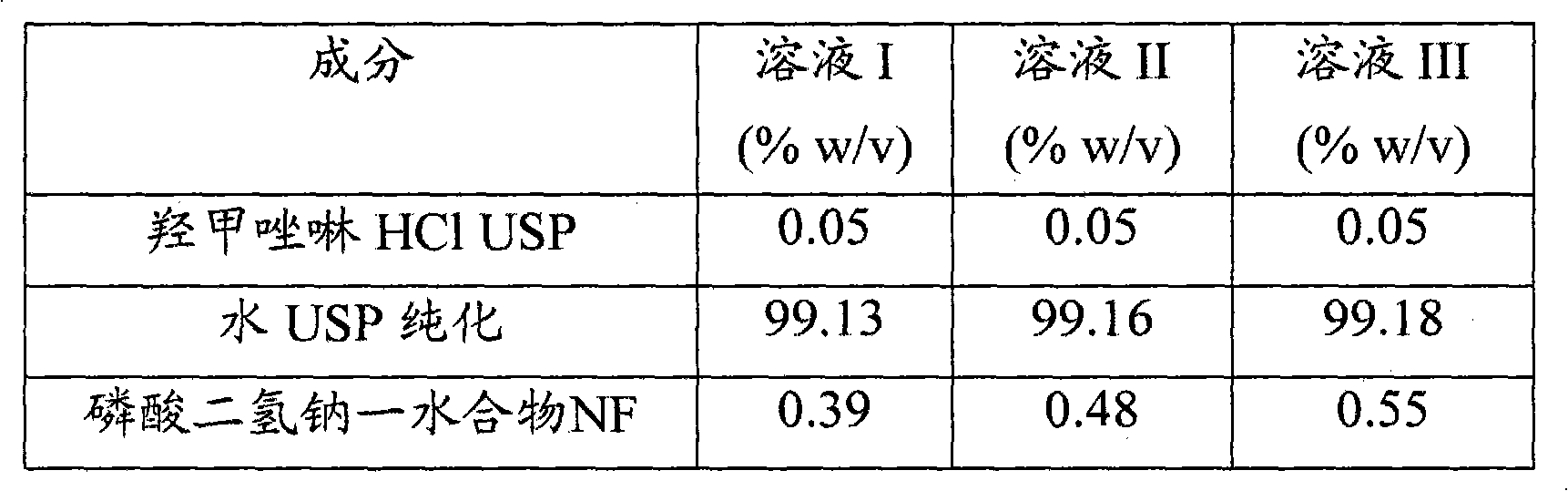
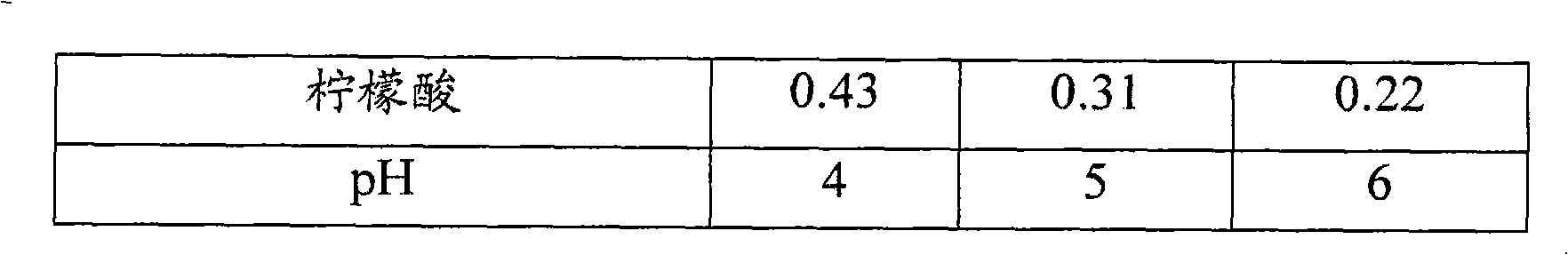
[0052] Using the Mcllvaine buffer system (Solutions I-III), 0.05% (w / v) oxymetazoline HCl solutions with pH ranging from 4 to 6 were prepared. The compositions used for the test solutions are listed in Table 1. At each pH level, 0.05% oxymetazoline HCl solutions containing either povidone 29-32 (approximately 3% w / v) or PEG 1450 (approximately 5% w / v) were also prepared (solutions IV-VIII) .
[0053] The compositions are prepared by conventional means by intimately mixing the ingredients, at room temperature or elevated temperature, in order for the ingredients to achieve their solubility where appropriate.
[0054] Table 1: Composition of Oxymetazoline HCl Solutions Prepared with Mcllvaine Buffer (pH Range: 4-6)
[0055]
[0056]
Embodiment 2
[0058] Photostability of the example embodiments was tested according to the method described in ICH Harmonized Tripartite Guidelines Stability Testing: Photostability Testing of New Drug Substances and Products sex. Each sample used for the photostability study was placed in a sealed quartz container and exposed to the exposure required by the ICH photostability guidelines (total exposure was 2.4 million lux hours and the integrated amount was close to UV energy for 400 Wh / m2) twice.
[0059] result
[0060] Table 2: Photostability of Oxymetazoline HCl in Mcllvaine buffer (pH range: 4-6)
[0061]
[0062] Table 3: Photostability of Oxymetazoline HCl in Mcllvaine buffer (pH range: 4-6) with addition of povidone K29-32 (approximately 3% w / v).
[0063]
[0064] Table 4: Photostability of Oxymetazoline HCl in Mcllvaine buffer (pH range: 4-6) with addition of PEG 1450 (about 5% w / v).
[0065]
[0066] All observed oxymetazoline HCl degradation peaks were calculated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com