Novel 18F labeled substituted benzimidazole compounds, preparation method thereof and PET tumor imaging application
A technology of benzimidazole and labeling method, applied in directions such as radioactive carriers, to achieve the effects of high labeling rate, short labeling time, and high tumor uptake
Inactive Publication Date: 2011-01-26
BEIJING NORMAL UNIVERSITY
View PDF2 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
And related researches are rarely reported at home and abroad, which also makes this invention highly innovative and has good application prospects.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
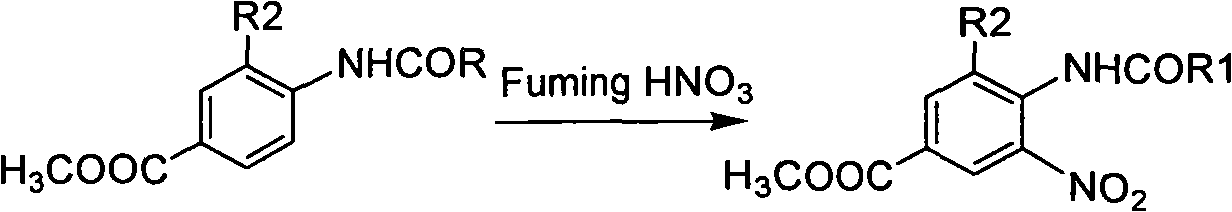
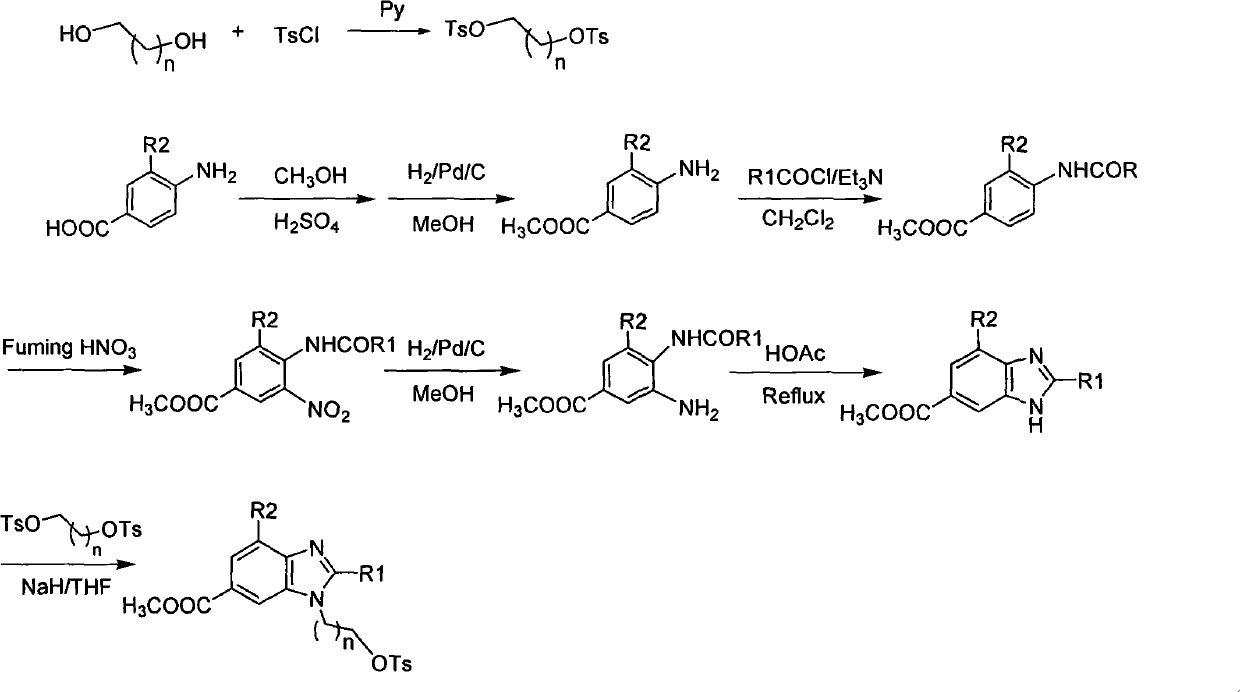
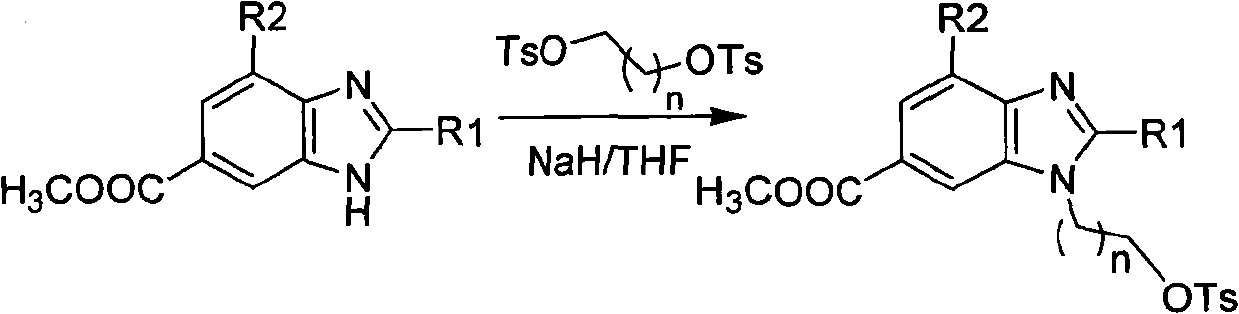
The invention provides novel 18F labeled substituted benzimidazole compounds, which are characterized in that: one end of each compound contains an 18F substituted alkoxy structure, and the other end contains a 6-carboxyl / H benzimidazole structure; a substituent group R1, located on the site 2 of benzimidazole matrix, is hydrogen, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, benzyl, 2-methylthio ethyl and phenyl; a substituent group R2, located on the site 4 of benzimidazole matrix, is hydrogen, methyl and ethyl; and the structure of the compound is shown in formula A, wherein n is between 1 and 5, R2 is H, Me and Et, X is COOH and H. Experiments show that the compounds have high bioactivity such as fast serum removal, high serum stability and relatively low intake in tissues or organs such as liver and relatively high enrichment and slow removal rate in tumor cells, and therefore the compounds have relatively high tumor / background value and are favorable for PET tumor imaging. Meanwhile, the labeled precursor of the compounds is easy to synthesize and the labeling rate is extremely high; and such advantages indicate that the compounds have the tremendous potential to become PET tumor imaging agents.
Description
Technical field: The present invention relates to a new class of 18 The F-labeled substituted benzimidazole compound, its preparation method and its application as a tumor positron emission tomography (PET) molecular probe. Background technique: Positron emission tomography (PET) is a powerful molecular imaging technique that is rapidly developing to study physiological processes such as substance metabolism, receptor binding, and biochemical mechanisms in living tissues. Today, PET has been widely used in the early diagnosis and postoperative evaluation of tumors, which has great research value and market demand. Among the nuclides commonly used in PET imaging, 18 F has a longer half-life (t 1 / 2 =110min), and has a lower radiation dose and a shorter range to the tissue, and at the same time, its hydrogen-like properties will not cause obvious changes in the spatial structure of the labeled molecules. Therefore, yes 18 The study of F-labeled PET imaging agents has beco...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K51/04A61K101/02
Inventor 齐传民王潇张淑婷贺勇刘航许荆立李桂霞冯曼
Owner BEIJING NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com