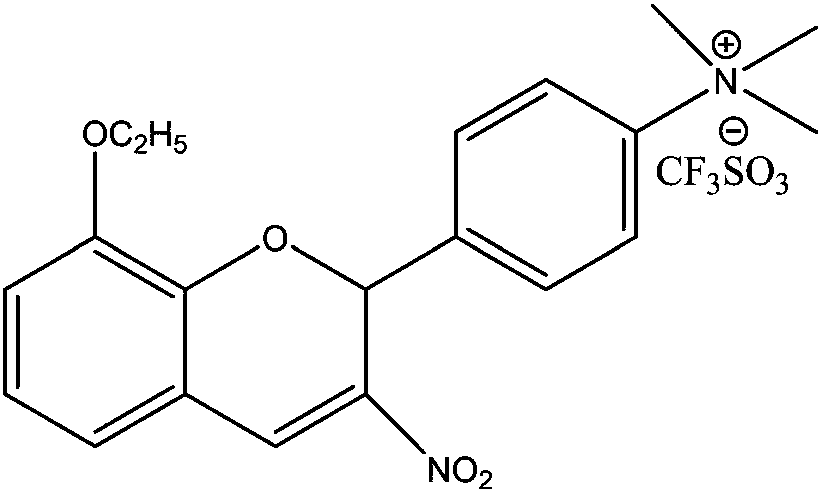
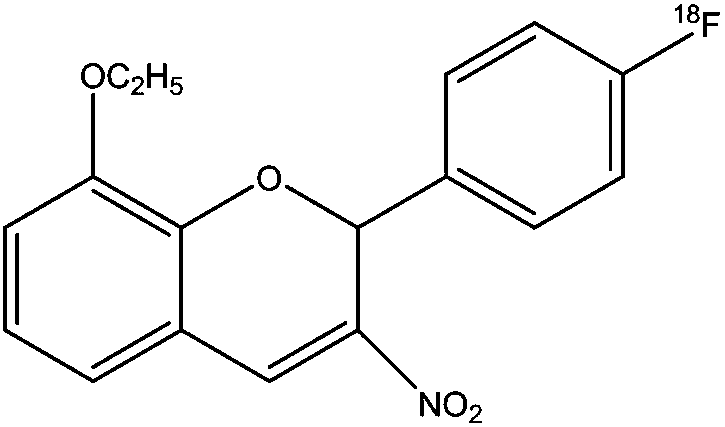
18f-labeled pi3k/akt signaling pathway inhibitor s14161 and its preparation method and application
A labeling and reaction technology, applied in the field of medicine, achieves the effects of simple and easy-to-control preparation process, mild reaction conditions, and simple and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
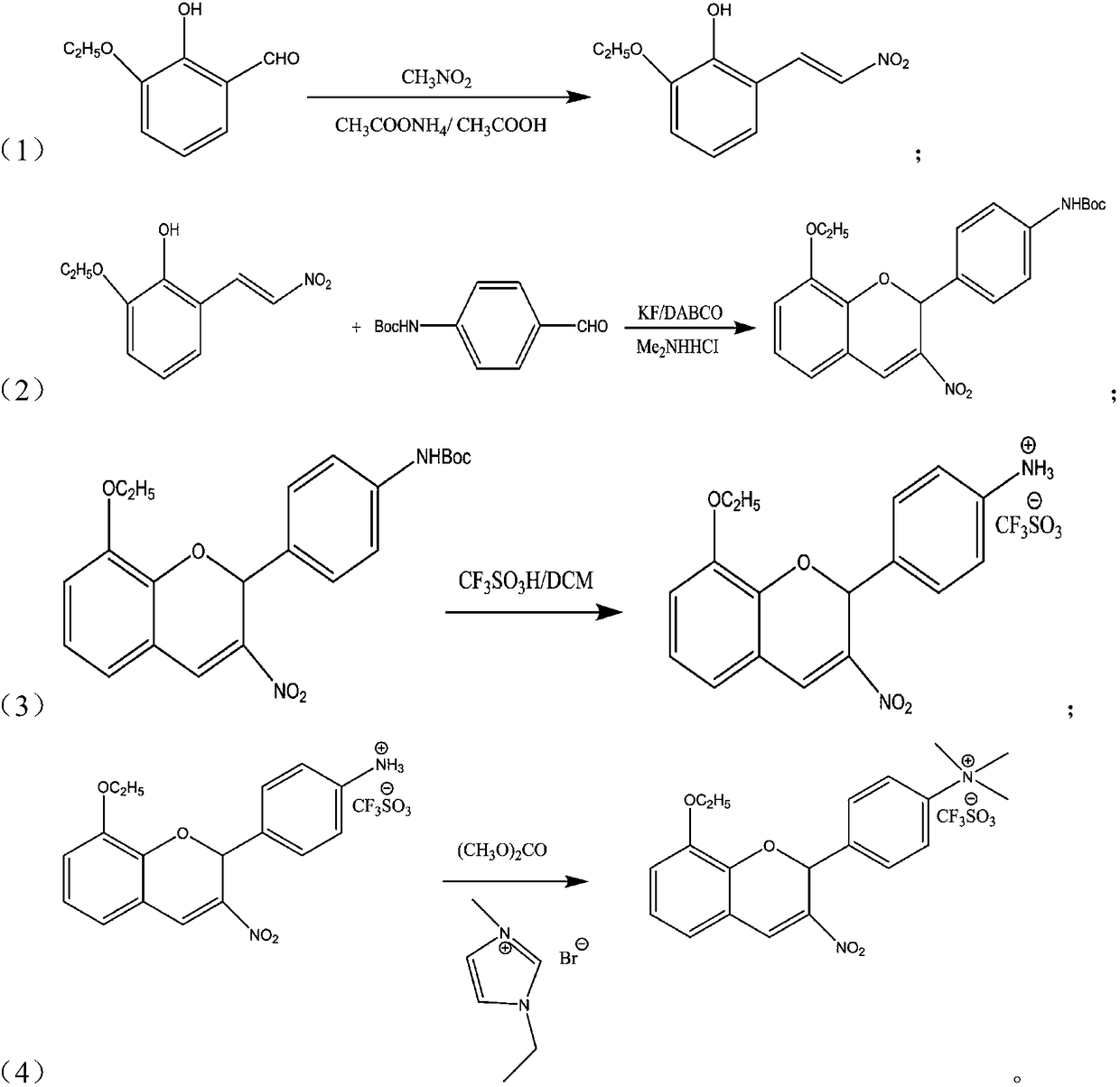
[0029] Embodiment 1: Synthesis of 2-hydroxyl-1-ethoxy-3-(2-nitrovinyl)benzene
[0030] Add 1.1g of ammonium acetate and 4.64ml of glacial acetic acid to the four-necked flask successively, stir, after fully dissolving, add 1mmol of 2-hydroxy-1-ethoxy-3-formylbenzene and 4mmol of nitromethane in sequence , heated to reflux, and reacted for 5 hours. After the reaction was monitored by TLC, the reaction solution was cooled to room temperature, then poured into crushed ice, stirred for 0.5 hours, and filtered with suction to obtain an orange crystalline compound, namely 2-hydroxyl-1-ethoxy Base-3-(2-nitrovinyl)benzene; purity 95%, yield 93%. ESI-MS(m / z):209(M + ); 1 H NMR (DMSO-d 6 ,,400MHz), δ:13.75(s,1H,OH),8.08(d,1H,J=7.85Hz,Ar-CH),8.19(d,1H,J=7.89Hz,CHNO 2 ), 6.61-7.15 (m, 3H, Ar-H), 4.09 (m, 2H, CH 2 O),1.4(t,3H,CH 3 CH 2 -), 13 CNMR (CDCl 3 ,125MHz) δ: 151.3, 148.1, 137.5, 138.8, 121.2, 120.1, 117.5, 114.9, 63.2, 14.5.
[0031]
Embodiment 2
[0032] Example 2: Synthesis of 8-ethoxy-2-(4-tert-butoxycarbonylaminophenyl)-3-nitro-2H-chromene
[0033] Add 10mmol of 2-hydroxyl-1-ethoxy-3-(2-nitrovinyl)benzene, 12mmol of 4-tert-butoxycarbonylaminobenzaldehyde, 1mmol of anhydrous potassium fluoride, 5mmol Triethylenediamine (DABCO), 10 mmol of dimethylamine hydrochloride, 72.6 ml of toluene, the mixture was heated to 110 ° C, and stirred for 12 h. Dichloromethane and water were added to the reaction solution, the organic phase was separated, dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 8-ethoxy-2-(4-tert-butoxycarbonylaminophenyl)-3-nitrate Base-2H-chromene, the yield is 70%, ESI-MS (m / z): 368 (M + ); 1 H NMR (DMSO-d 6 ,,400MHz)δ:9.79(s,1H,NH),7.88(s,1H,NO 2 CCH), 6.61-7.58 (m, 7H, Ar-H), 5.6 (s, 1H, OCH), 4.13 (m, 2H, CH 2 O),1.48(d,9H,3×CH 3 ), 1.39(t,3H,CH 3 CH 2 -), 13 CNMR (CDCl 3 ,125MHz)δ:147.1,81.3,148.9,145.3,136.1,115.1,136.9,121.3,121.8,120.3,127.6,114.1,121.3,127.5,...
Embodiment 3
[0035] Example 3: Synthesis of the triflate salt of 8-ethoxy-2-(4-aminophenyl)-3-nitro-2H-chromene
[0036] Add 10mmol of 8-ethoxy-2-(4-tert-butoxycarbonylaminophenyl)-3-nitro-2H-chromene, 15mmol of trifluoromethanesulfonic acid, 59.3ml of dichloromethane into the reaction flask , stirred at room temperature for 0.5h, after the completion of the reaction monitored by TLC, the solvent was evaporated to dryness to obtain trifluoromethanesulfonic acid of 8-ethoxy-2-(4-aminophenyl)-3-nitro-2H-chromene. The rate is 90%. ESI-MS(m / z):459(M + ); 1 H NMR (DMSO-d 6 ,,400MHz)δ:6.63-7.56(m,7H,Ar-H),7.35(s,3H,NH 3 ),7.65(s,1H,NO 2 CCH), 5.66(s,1H,OCH), 4.11(m,2H,CH 2 O),1.39(t,3H,CH 3 CH 2 -). 13 CNMR (CDCl 3 ,125MHz) δ: 148.3, 82.0, 149.1, 145.8, 133.1, 115.5, 141.2, 121.5, 121.4, 120.3, 127.5, 114.3, 121.5, 127.3, 121.6, 28.6, 64.3, 14.9.
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com