Novel side chain of 25-hydroxyitamin D2 series medicines and preparation method thereof
A technology of hydrocarbon group and amino group, applied in the field of preparation of common side chains, can solve the problems of low yield, limitation, difficult synthesis and purification, etc., and achieve the effects of high yield, easy product and short route.
Active Publication Date: 2011-01-26
SHANGHAI HAOYUAN CHEMEXPRESS
View PDF2 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Compound 5 not only has a low yield when it is used for drug synthesis, but also the synthesis and purification of compound 5 itself is difficult, and its synthesis process also causes some limitations for the protective group R functional group
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
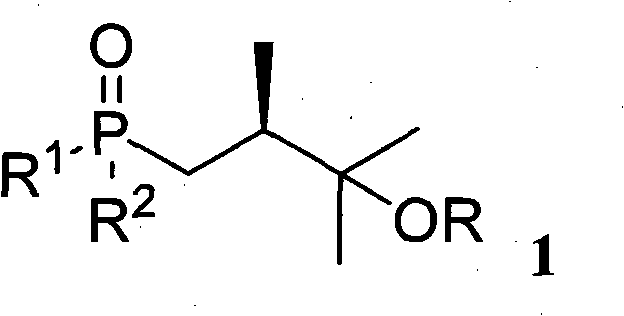
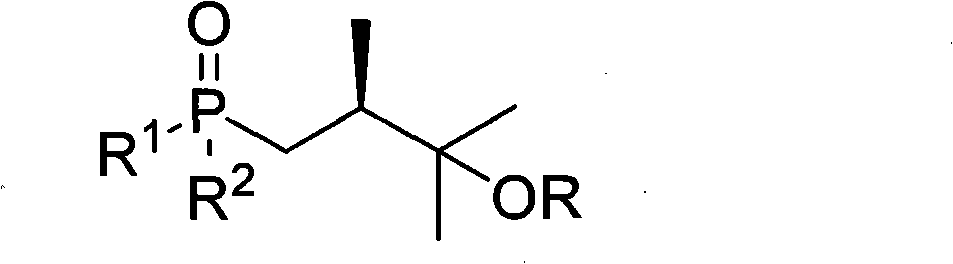
The invention relates to a preparation of organic compounds, in particular to a preparation method of a common side chain of a series of 25-hydroxyitamin D2 medicines. The structure of the compounds is represented in the formula 1. The compounds have wide application and are more stable than the traditional compounds with the same application, so that the compounds are convenient to storage and use. The method for synthesizing the target compounds (1) has high yield, short synthesis route and easily purified products.
Description
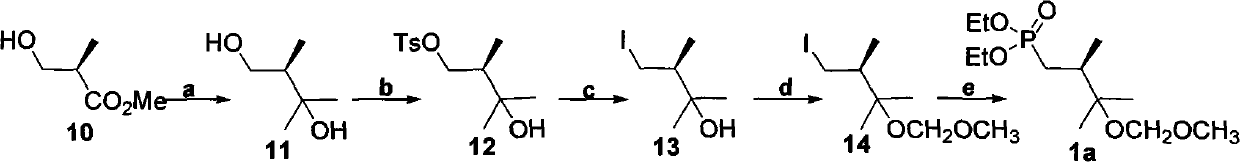
technical field The present invention relates to a method for preparing an organic compound, specifically a method for preparing a series of common side chains of 25-hydroxyvitamin D2 drugs, the structure of which is shown in Formula 1 below. Formula 1 Among them, R is a hydrogen atom or -SiR 3 R 4 R 5 or -R 3 or -R 6 OR 4 or -C(=O)R 3 , where R 1 , R 2 , R 3 , R 4 , R 5 , R 6 are hydrocarbyl or hydrocarbyloxy or amino or hydrocarbyl-substituted amino, and they may be the same or different. Background technique Formula 2 Formula 3 A series of 25-hydroxyvitamin D2 drugs shown in formula 2 have a common side chain, and these drugs can be synthesized from compound 1 through the process shown in formula 3. These drugs include 25-hydroxyvitamin D2, 1a, 25-dihydroxyvitamin D2, paricalcitol, and others. These drugs are regulators of calcium and phosphorus homeostasis in animals and humans 1,2 . Recent studies have also identified their activity in cell ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F9/40C07F9/655C07F9/53
Inventor 高强薛吉军郑保富刘荣
Owner SHANGHAI HAOYUAN CHEMEXPRESS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com