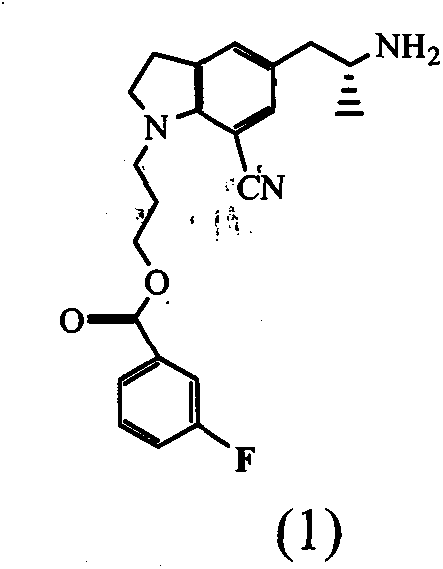
Indoline compound with optical activity and preparation method thereof
A compound, the technology of cyanoindoline, which is applied in the preparation of the intermediate compound, the intermediate compound field of synthesizing the drug silodosin for the treatment of benign prostatic hyperplasia, can solve the problem that the multi-step reaction yield is low and is not suitable for industrial production , L-Phenylglycinol is expensive and other problems, to achieve the effect of high asymmetric induction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
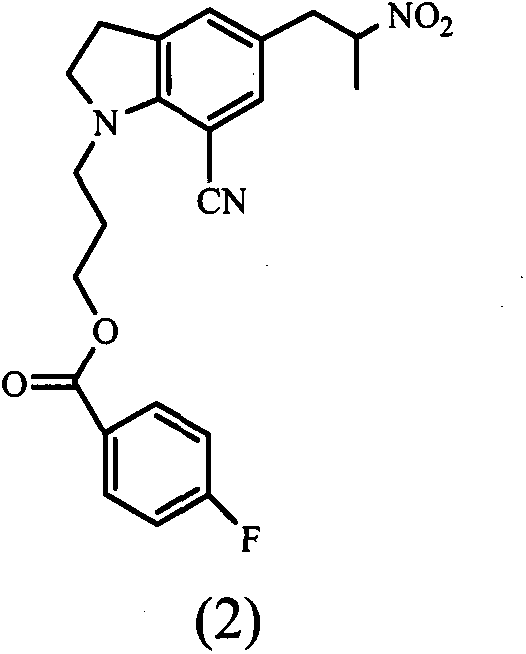
[0041] Embodiment 1: the preparation of compound (2)
[0042] Referring to the compound (2) synthetic route diagram provided in the summary of the invention
[0043] A: Preparation of compound (9)
[0044] 264 grams of 4-fluorobenzoic acid was dissolved in 550ml of DMF. Put 375ml of bromochloropropane, 265ml of triethylamine, and 300ml of DMF in a reaction flask, add 4-fluorobenzoic acid dropwise at room temperature, and react at room temperature for 12h. Add water, extract with ethyl acetate, wash the organic layer with saturated aqueous sodium bicarbonate solution and brine, dry the organic layer with anhydrous sodium sulfate, remove the solvent under reduced pressure to obtain oil compound (8)
[0045] The mass spectrometry of the oil showed that the molecular ion peak [M+1] was 217.
[0046] Compound (8) 391 grams, diisopropylethylamine 473ml, indoline 182ml) and DMF1600ml, stirred at 105°C for 16 hours. Added water, extracted with ethyl acetate, washed with saturated a...
Embodiment 2
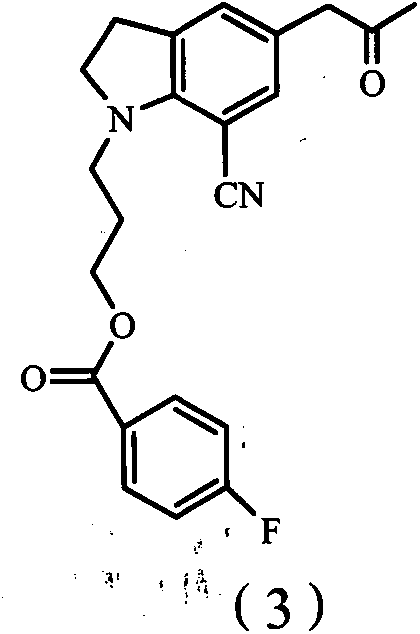
[0066] Embodiment 2: the preparation of compound (3)
[0067] 100 g (0.243 mol) of compound (2) was dissolved in 450 ml of DMF, and at 0-5°C, 58 ml (0.389 mol) of DBU was added, then 125 ml (0.738 mol) of triethylchlorosilane was added dropwise at low temperature, and the reaction 3 hours, then dropwise added 30% H 2 o 2 41 grams (0.362mol), after the dropwise addition, react for 1 hour. The reaction solution was added dropwise to water, and extracted with ethyl acetate to obtain 97.27 g of compound (3) oil
[0068] The mass spectrum of the oil showed that the molecular ion peak [M+1] was 381; the HPLC purity was 93%.
Embodiment 3
[0069] Embodiment 3: the preparation of compound (4)
[0070] Compound (3) 97.27 grams, dissolved in 500mlTHF, added R-(+)-α-phenylethylamine 29.5 grams (0.243mol), 0.4 grams of PtO 2 , AcOH14.5ml (0.243mol), transferred to hydrogenation kettle, 40-70 ℃, hydrogen pressure 2-5atm. Platinum oxide was filtered out, concentrated under reduced pressure to obtain an oily product, heated and dissolved in ethyl acetate, extracted with water, dried over anhydrous sodium sulfate, filtered, and concentrated to remove the solvent to obtain 119 g of an oily product (greater than the theoretical amount). This gave a mixture with a diastereomeric ratio of 6:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com