Device for promotion of hemostasis and/or wound healing
A technology of composition and thrombin, applied in the direction of application, drug combination, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0643] Example 1: Determination of the reconstitution rate of gelatin-based sponges
[0644] The purpose of this method is to determine the rate of reconstitution of gelatin-based sponges. The method involves soaking the sponge, followed by squeezing it. The emergence of the sponge's natural shape was monitored as a function of time, and the time required until the sponge reached its natural shape was referred to as the reconstitution time.
[0645] The method comprises the steps of:
[0646] 1. Cut a suitable piece of absorbent gelatin-based sponge approximately 1x1 cm and soak it in water at room temperature.
[0647]2. Take the sample out of the water, and squeeze it until it is flat and no more air bubbles or water droplets can be squeezed out.
[0648] 3. Place the sample in a beaker filled with water at room temperature and measure the time (in seconds) for the sample to acquire its original size and shape.
[0649] 4. Repeat the test twice and report the average o...
Embodiment 2
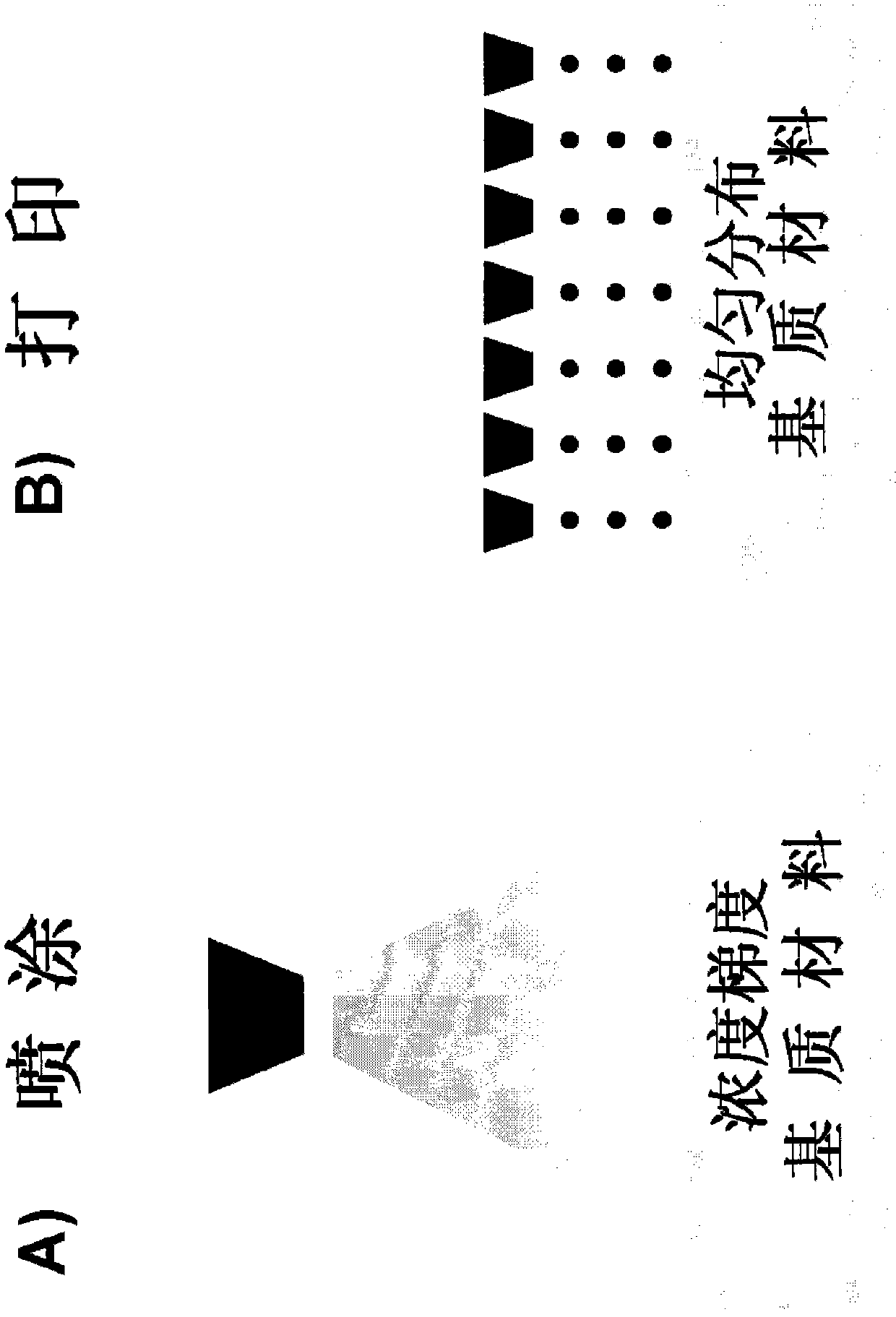
[0651] Examples of possible printing media or compositions for printing on substrate surfaces are given here. In this example, the bioactive agent contained in the pharmaceutical composition is thrombin and the matrix is a collagen-based sponge.
[0652] Print Media: Sterile MQ-water, sterile saline or another suitable sterile aqueous solvent adjusted to 10 cps with a suitable biocompatible viscosity enhancer such as gelatin. Thrombin is reconstituted to the appropriate concentration in media. This concentration should be adjusted so that the final concentration of thrombin produces 30 IU / cm on the surface of the substrate to be printed 2 . The pH was maintained in the physiological range, while the temperature was maintained at room temperature (approximately 25°C). Suitable surfactants may be added to the medium, if desired.
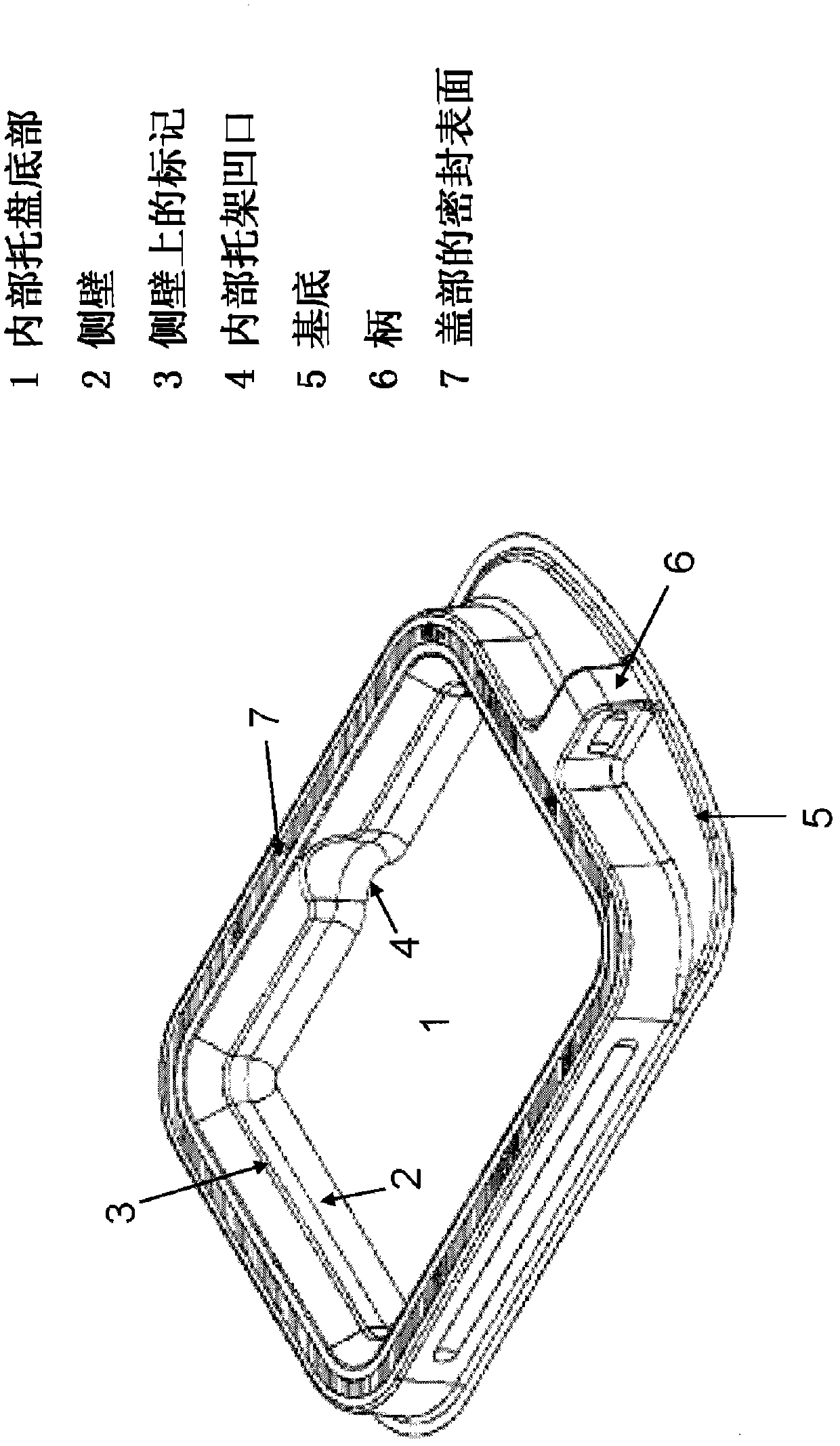
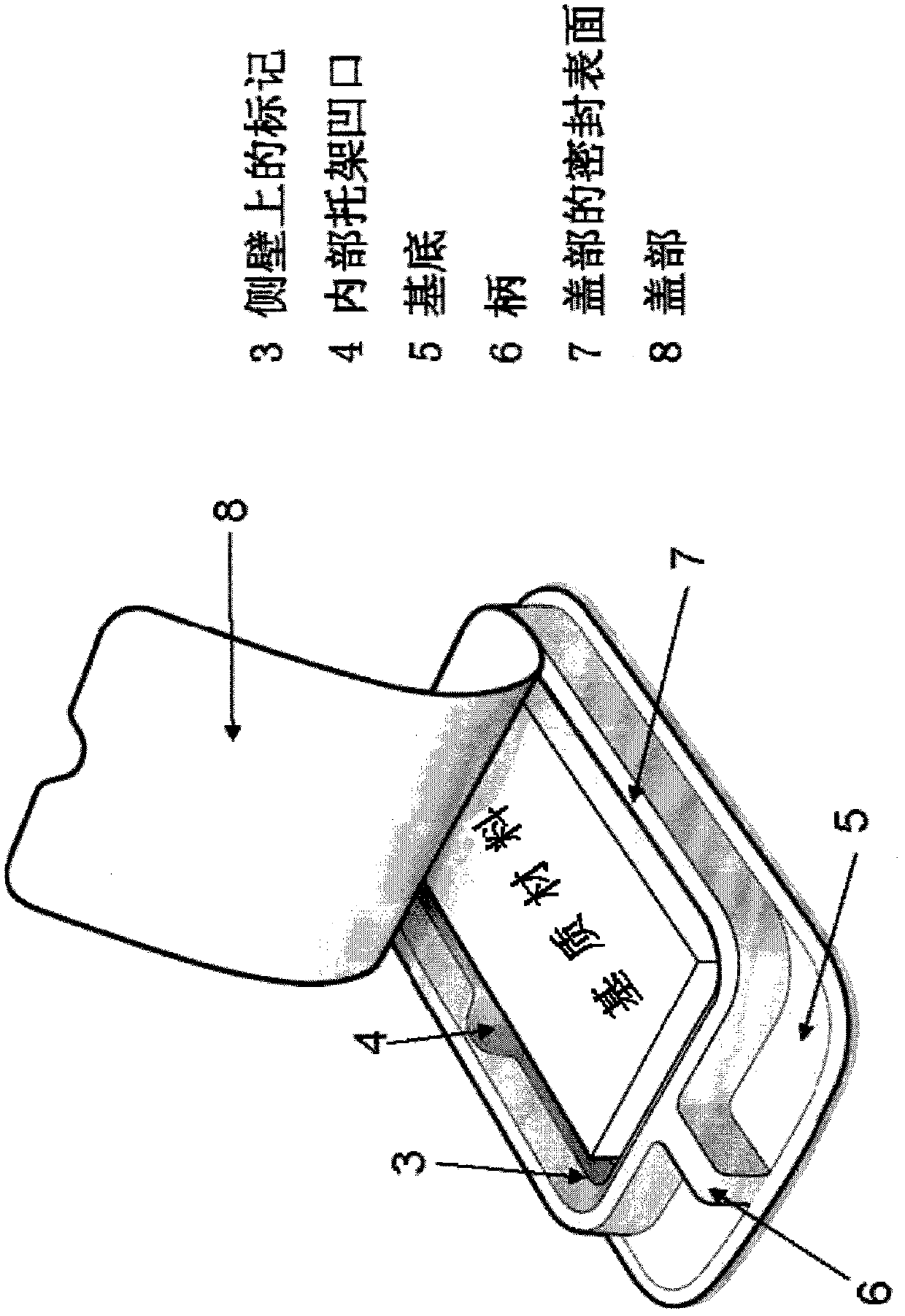
[0653] In one embodiment, the matrix of choice is a gelatin-based sponge, such as the commercially available Spongostan 、Surgifoam or Surgif...
Embodiment 3
[0655] Example 3: Hemostatic effect of substrates printed with thrombin
[0656] A porcine model has been used to test the hemostatic effect of different amounts of thrombin printed on a substrate (Surgifoam™; Johnson and Jonhson). Comparison of wet and dry application of thrombin matrix. For dry application, when indicated in the graph, for low concentrations of thrombin (20, 10 or 5 IU / cm 2 ), thrombin was printed on the substrate. The matrix printed with thrombin with dry application had a surprisingly effective hemostatic effect (in terms of hemostasis time) at low thrombin concentrations (see figure below).
[0657]
[0658] Legend: The efficacy of Surgifoam with thrombin applied by inkjet printing was tested in the porcine spleen model and compared to standard wet application of Surgifoam+thrombin. The result is the median + / - max / min. Surgifoam is applied wet or dry. For wet application, 4cm 2 Surgifoam in 800ul saline, 800ul 1000IU / ml (~200IU / cm 2 ) standa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap