Preparation method and application of deuterated drugs
A technology of deuterium and medicine, applied in the field of medicine and medicine, can solve the problems of limited technical approaches and means of chemical bonds and hydrogen bond energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
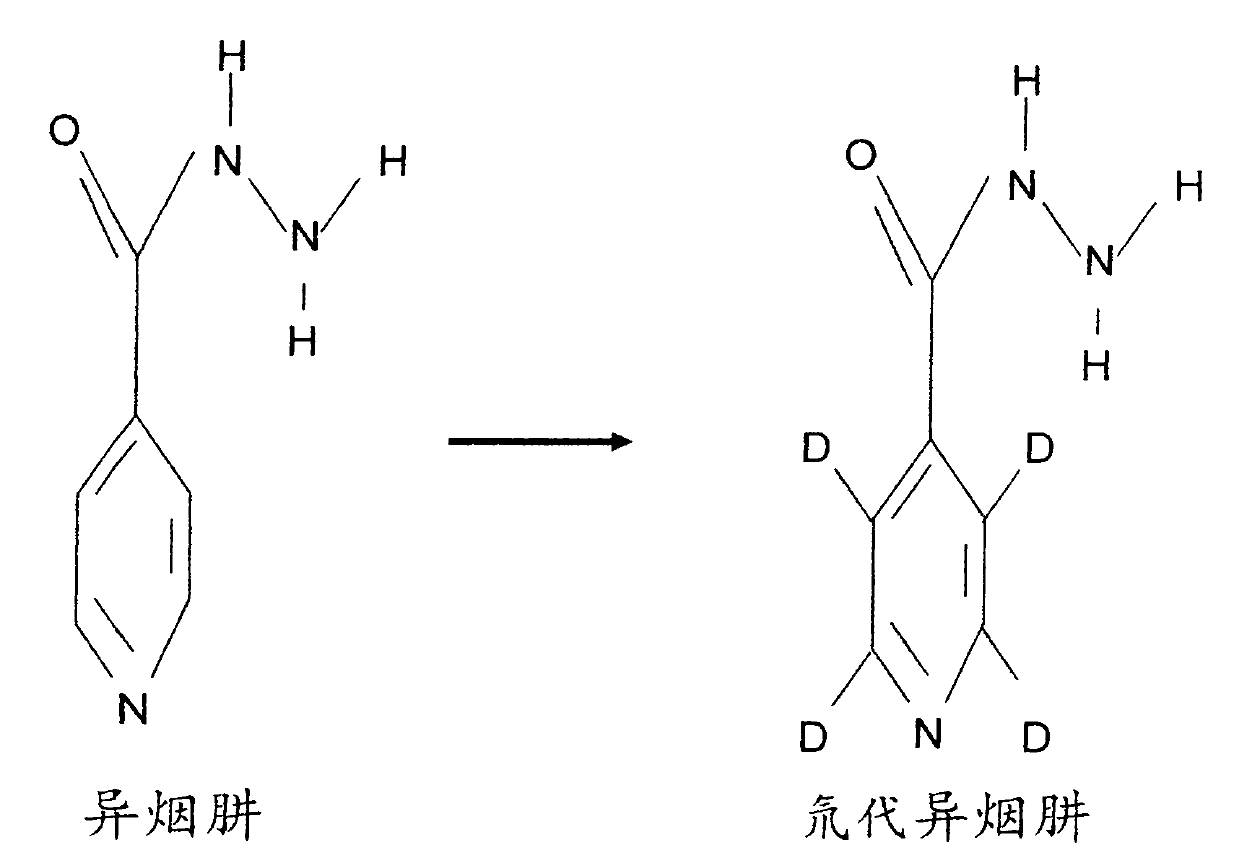
[0034] 1. Preparation of deuterated isoniazid:
[0035] Isoniazid was once a widely used anti-tuberculosis drug, which has a bactericidal effect on Mycobacterium tuberculosis, and Mycobacterium tuberculosis is prone to develop drug resistance to this product. Since the current clinical tuberculosis pathogenic strains are mainly drug-resistant strains, isoniazid is no longer used alone, but in combination with other anti-tuberculosis drugs. Large doses of isoniazid can easily cause liver damage, and combined use of rifampicin can significantly increase liver damage. Therefore, increasing the sensitivity of isoniazid to Mycobacterium tuberculosis and reducing the dosage has obvious clinical value. Therefore, Isoniazid was selected for deuterium substitution to develop new anti-tuberculosis drugs.
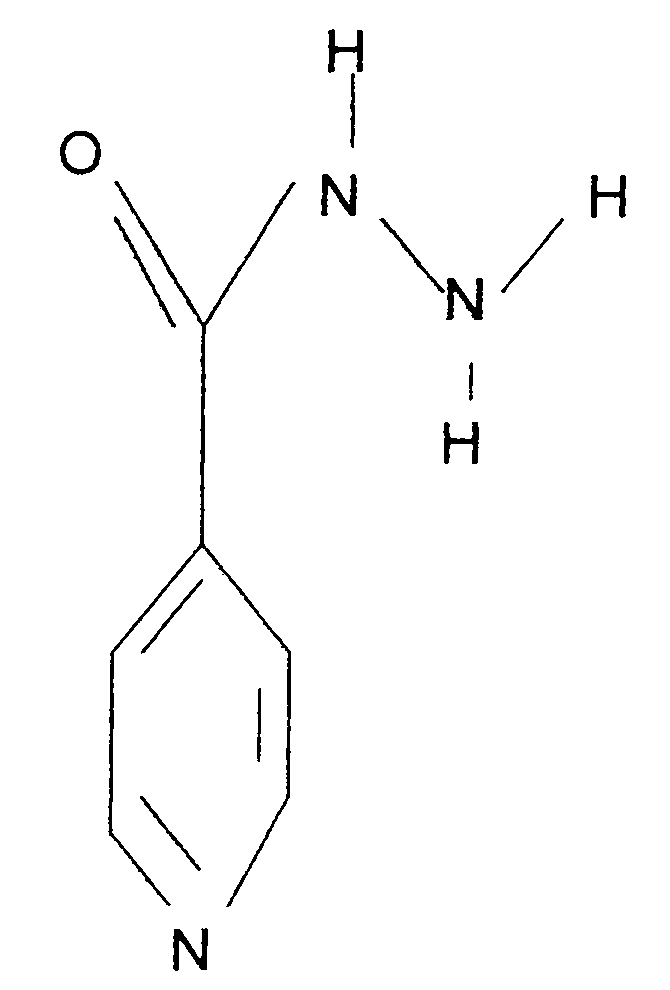
[0036] Chinese name: Isoniazid;
[0037] English name: Isoniazid;
[0038] Chemical name: 4-pyridinecarbohydrazide
[0039] CAS NO: 54-85-3
[0040] Molecular formula: C 6 h 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com