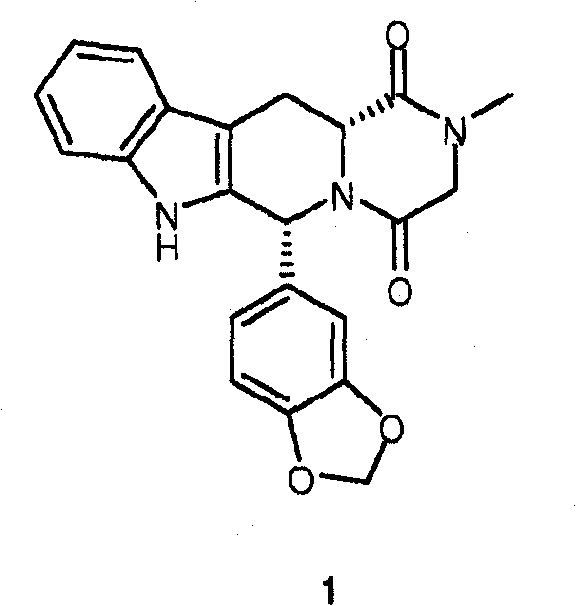
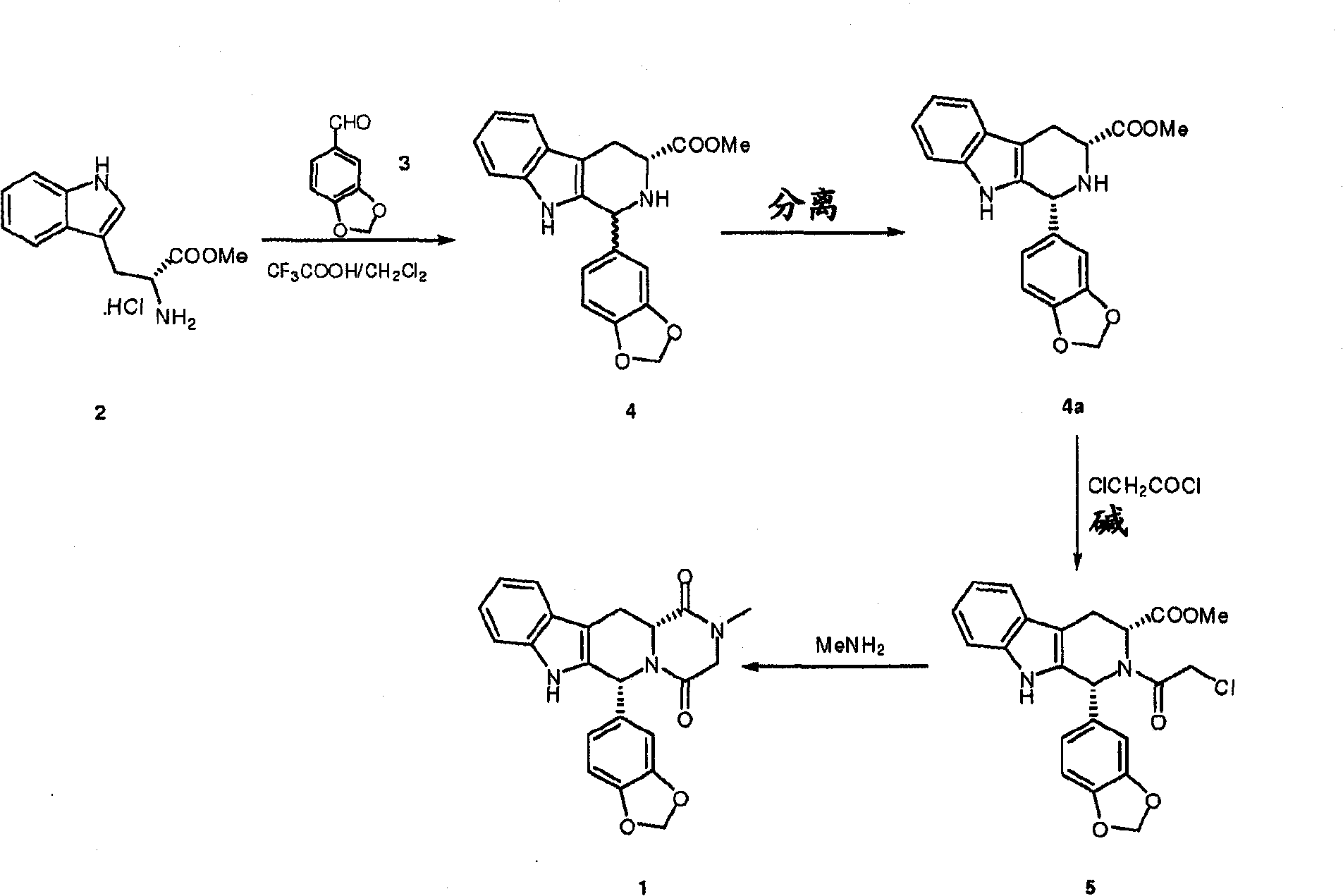
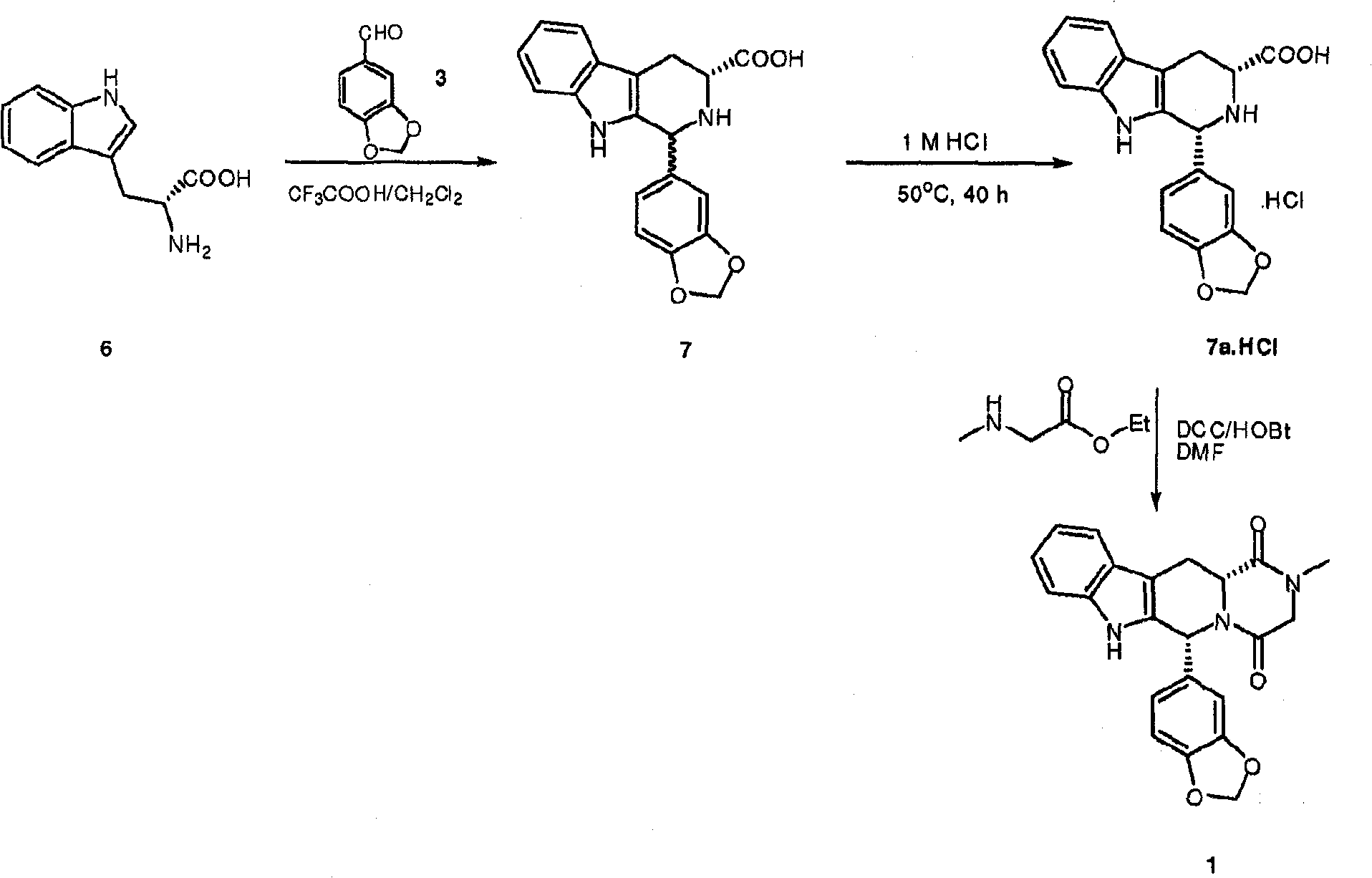
Conversion of tryptophan into beta-carboline derivatives
A tryptophan and carboline technology, applied in organic chemistry and other fields, can solve problems such as shortening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1R,3R)-1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carba Preparation of hydrochloride
[0052] D-Tryptophan (7.96 g, 39 mmol) was suspended in THF (60 ml), and the flask was purged with nitrogen. Piperonal (6.46 g, 43 mmol) was added and the mixture was stirred at room temperature for 5 minutes. Concentrated aqueous hydrochloric acid (4ml) was added and the solution was heated to reflux. The resulting slurry was refluxed for 3 days. The suspension was then cooled to 0-5°C and the product was filtered off, washed with cold THF and dried under vacuum at room temperature for 18 hours to yield 9.6 g of product (98% area).
Embodiment 2
[0054] (1R,3R)-1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carba Preparation of hydrochloride
[0055] D-Tryptophan (2 g, 10 mmol) was suspended in acetonitrile (150 ml), and the flask was purged with nitrogen. Piperonal (1.65 g, 11 mmol) was added and the mixture was stirred at room temperature for 5 minutes. Concentrated aqueous hydrochloric acid (1 ml) was added and the mixture was heated to reflux. The resulting slurry was refluxed for 3 days. The suspension was then cooled to 0-5°C and the product was filtered off, washed with cold water and dried under vacuum at 40°C for 18 hours to yield 3.1 g of product (99.8% area)
Embodiment 3
[0057] (1R,3R)-1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carba Preparation of hydrochloride
[0058] Suspend D-tryptophan (4g, 19.6mmol) in 1,4-bis Alkanes (40ml), the flask was purged with nitrogen. Piperonal (3.25 g, 21.7 mmol) was added and the mixture was stirred at room temperature for 5 minutes. Concentrated aqueous hydrochloric acid (2ml) was added and the solution was heated to 65°C. The resulting slurry was stirred at 65°C for 16 hours. The suspension was then cooled to 0-5°C, the product was filtered off and washed with 1,4-bis Washed with alkane and dried under vacuum at 40°C for 18 hours, yielding 3.6 g of product (99.7 area%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com