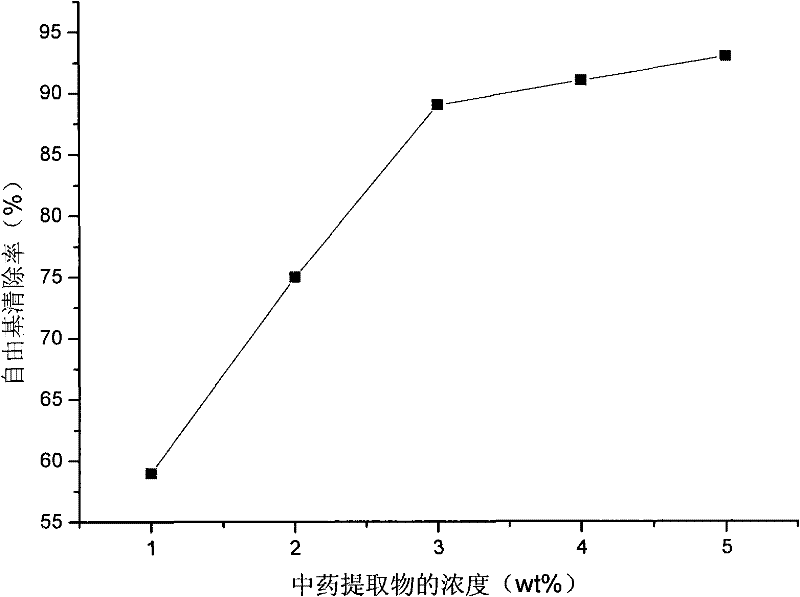
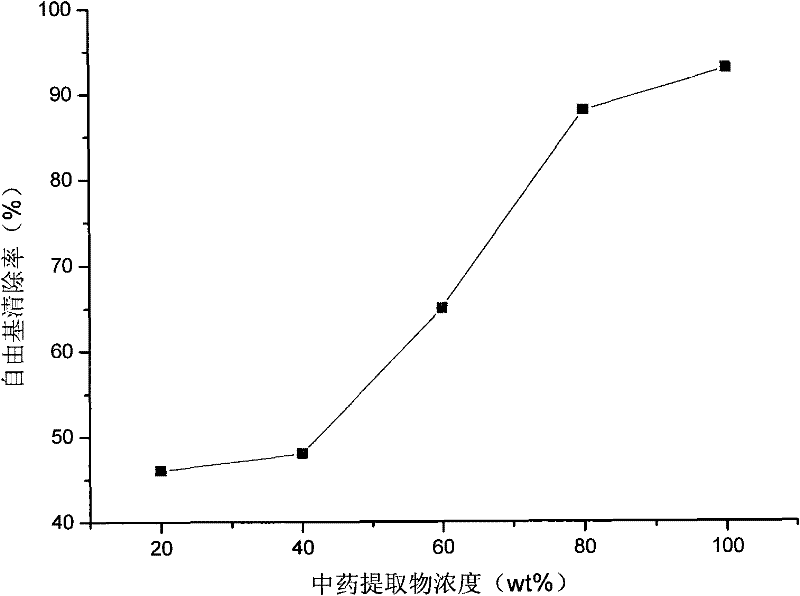
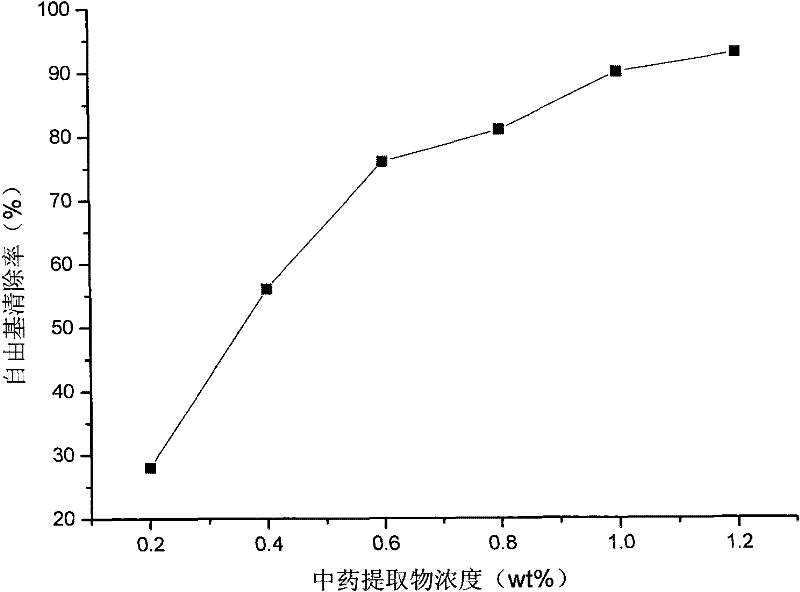
Skin care composition with wrinkle removing and resisting function and preparation and preparation method thereof
A skin care composition and composition technology, applied in skin care preparations, pharmaceutical formulations, cosmetic preparations, etc., can solve the problems of insignificant anti-wrinkle and anti-wrinkle effects, high ion content, and low addition amount, and achieve anti-wrinkle effects Visible, less skin-irritating, texture-improving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation of embodiment 1 Chinese medicine extract of the present invention
[0081] (1) Take each bulk drug according to the following components and dosage:
[0082] Astragalus 17g, Ophiopogon japonicus 13g, Pseudostellaria 15g, Salvia miltiorrhiza 10g, licorice 5g.
[0083] (2) Add the bulk drug to deionized water, start timing from the moment when the liquid boils, and boil for 45 minutes; the mass volume g / ml ratio of the bulk drug to deionized water is 1:10, that is, add 600ml of deionized water;
[0084] (3) Stop heating, cool to 30°C, and filter with 200 mesh;
[0085] (4) The filtrate obtained in step (3) is vacuum filtered with diatomite, the fineness is 500 mesh, and the vacuum degree is 0.05-0.1MPa. The vacuum degree will fluctuate according to the change of the liquid flow rate, as long as it is within this range Yes, the Chinese medicine extract is finally prepared.
[0086] Measure the pH value, solid content and conductivity, and the measurement...
Embodiment 2
[0087] The preparation of embodiment 2 Chinese medicine extracts of the present invention
[0088] (1) Take each bulk drug according to the following components and dosage:
[0089] Astragalus 10g, Ophiopogon japonicus 30g, Pseudostellaria 10g, Salvia miltiorrhiza 20g, licorice 10g.
[0090] (2) Add the bulk drug to deionized water, start timing from the moment when the liquid boils, and boil for 30 minutes; the mass-volume ratio g / ml of the bulk drug to deionized water is 1:20, that is, add 1600ml of deionized water;
[0091] (3) Stop heating, cool to 45°C, filter with 100 mesh;
[0092] (4) The filtrate obtained in step (3) is vacuum filtered with diatomite, the fineness is 600 mesh, and the vacuum degree is 0.05-0.1MPa. The vacuum degree will fluctuate according to the change of the liquid flow rate, as long as it is within this range Yes, the Chinese medicine extract is finally prepared.
[0093] Measure the pH value, solid content, and conductivity, and the measurement...
Embodiment 3
[0094] The preparation of embodiment 3 Chinese medicine extract of the present invention
[0095] (1) Take each bulk drug according to the following components and dosage:
[0096] Astragalus 20g, Ophiopogon japonicus 10g, Pseudostellaria 25g, Salvia miltiorrhiza 15g, licorice 20g.
[0097] (2) Mix the raw material medicine with deionized water, start timing from the moment when the liquid boils, and boil for 90 minutes; the mass volume ratio of the raw material medicine and deionized water is 0.5:10, that is, add 1800ml of deionized water;
[0098] (3) Stop heating, cool to 50°C, and filter with 200 mesh;
[0099] (4) The filtrate obtained in step (3) is then subjected to diatomite vacuum filtration, the fineness is 700 mesh, the vacuum degree is 0.05-0.1MPa, and the vacuum degree will fluctuate according to the change of the liquid flow rate, as long as it is within this range Yes, the Chinese medicine extract is finally prepared.
[0100] Measure the pH value, solid cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com