Method for enriching glycopeptide and simultaneously enriching glycopeptide and phosphorylated peptide by using metal oxide
A technology for phosphorylating peptides and oxides, applied in the direction of peptides, etc., to achieve the effect of strong universality, no activation time, and convenient and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033]Preparation of sample solution: 1mg of α-casein was dissolved in 1mL of 50mM ammonium bicarbonate solution, and trypsin was added according to the mass ratio of α-casein and trypsin at a ratio of 1:40 (w / w), and then digested at 37°C. After reacting for 16 hours, formic acid with a final concentration of 0.5% (v / v) was added to terminate the enzymatic hydrolysis.
[0034] Dissolve 1 mg of human serum immunoglobulin and 1 mg of horseradish peroxidase in 100 μl of 50 mM ammonium bicarbonate containing 8 mol / L urea and shake for 3 hours. Thiothreitol (Dithiothreitol; DTT for short) solution was placed at 37°C for 2 h, and then 10 μL of iodoacetic acid (IAA) with a concentration of 50 mmol / L was added to the protein solution respectively, and stood at room temperature for 30 minutes in the dark. Dilute this solution ten times with ammonium bicarbonate, add trypsin, enzymolyze according to the mass ratio of protein to trypsin 1:40 (w / w), react at 37°C for 16 hours, and then a...
Embodiment 1
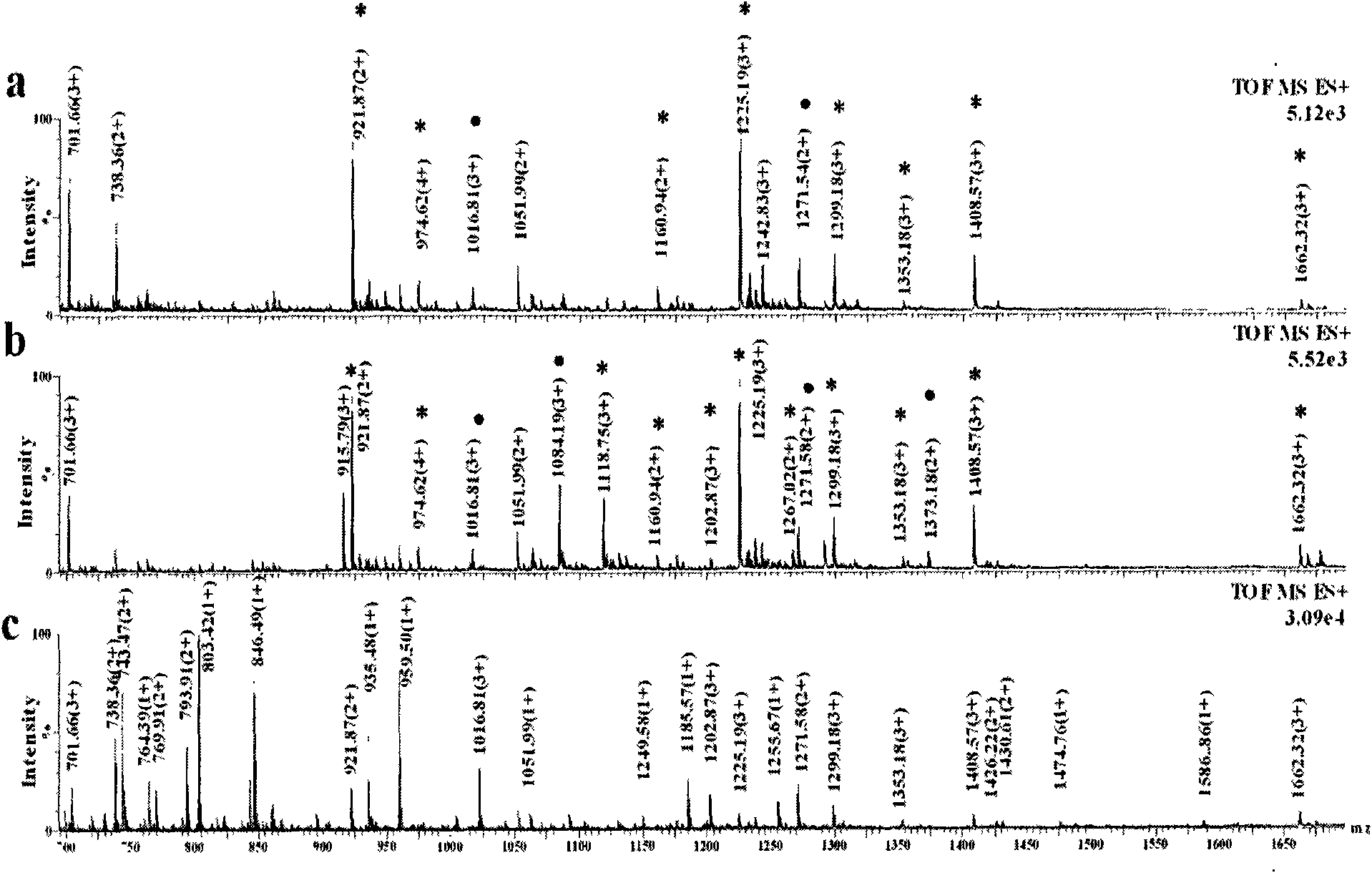
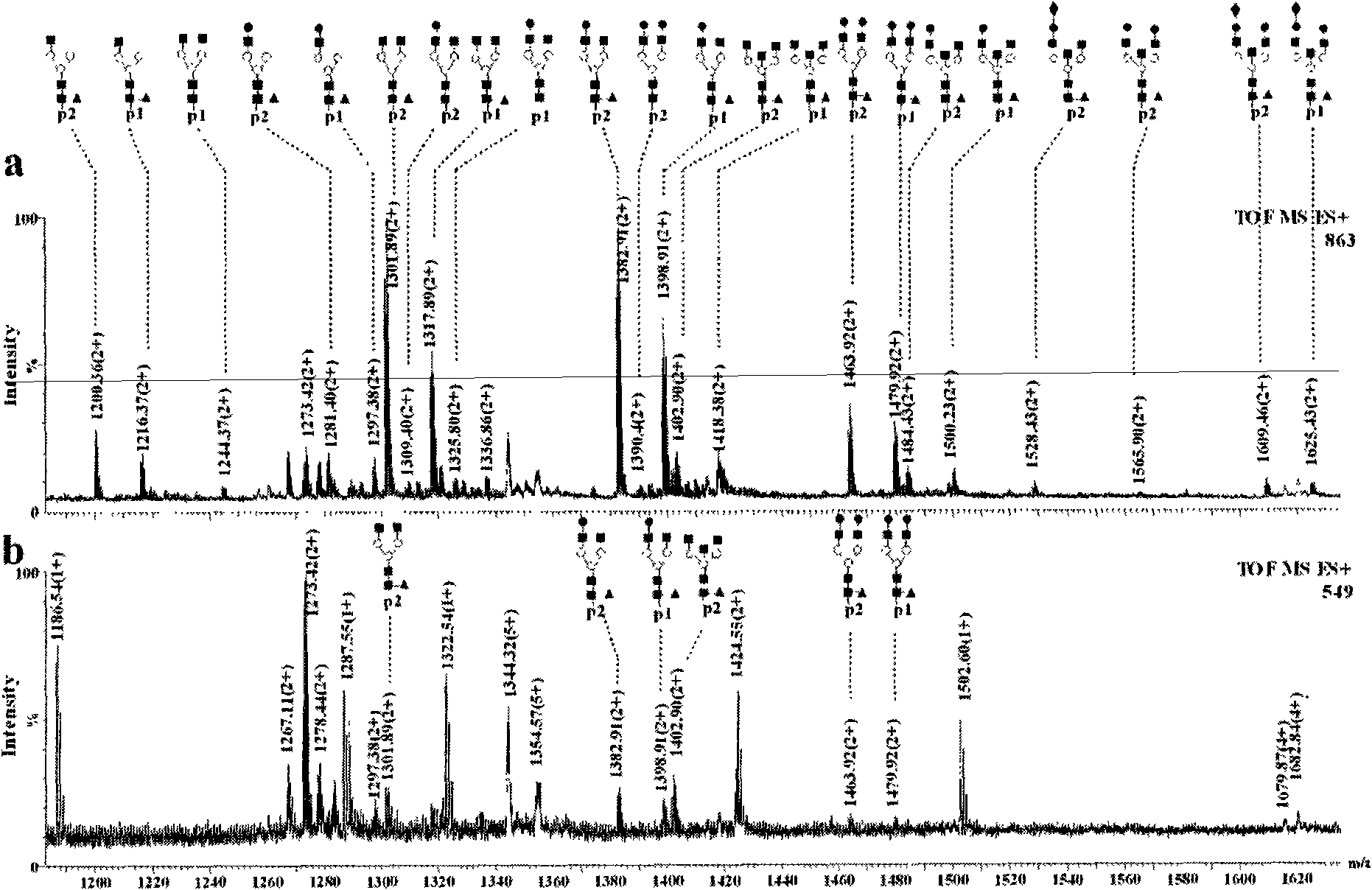
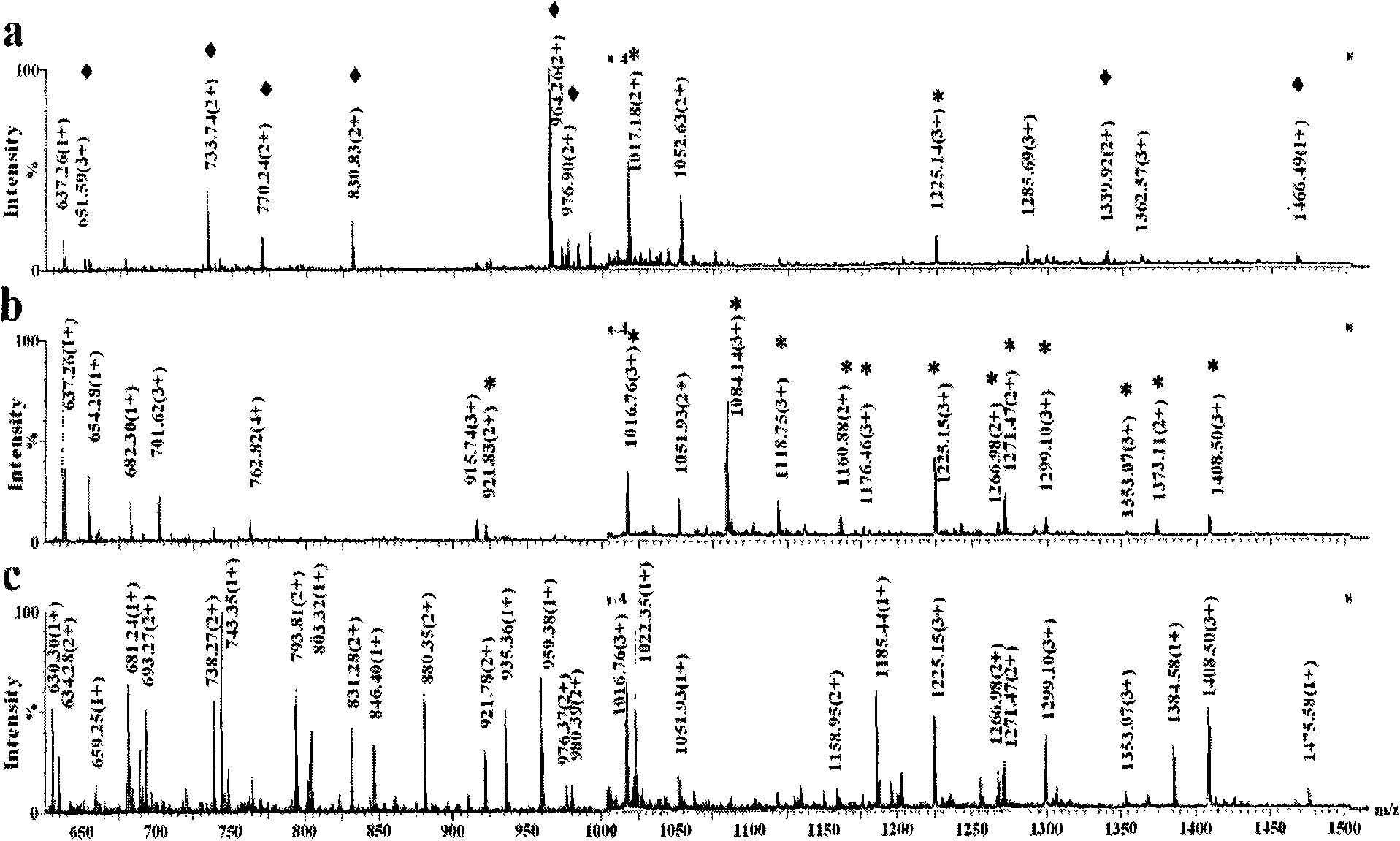
[0036] Titanium oxide enrichment of glycopeptides: 1 mg of titanium oxide (purchased from GL Science) was homogenized into a GELoader tip tube, and 3.3 μL (83.3 pmol) of horseradish peroxidase that had been neutralized to neutral with ammonia water The solution solution was loaded and washed three times with 30 μL of 50 mM ammonium bicarbonate solution, 30 μL of 0.1% formic acid solution for three times, and three times for 30 μL of acetonitrile aqueous solution containing 0.5% formic acid. 50% acetonitrile, and finally eluted with 10 μL of acetonitrile aqueous solution containing 5% formic acid in volume concentration, wherein the acetonitrile aqueous solution contains 50% acetonitrile in volume concentration, and the eluate was collected and analyzed by nanoESI-Q-Tof injection.
[0037] Comparison of spectra before and after enrichment figure 1 (c) and 1(a), it can be seen that the glycopeptides hydrolyzed by the standard glycoprotein horseradish peroxidase are effectively e...
Embodiment 2
[0039] The difference from Example 1 is that the titanium oxide used is self-made titanium oxide by sol-gel method [Patent No. 200810202077.1].
[0040] The preparation method of titanium oxide is as follows:
[0041] Step (1) Add 0.0024mol of dodecylamine, 100ml of absolute ethanol, 0.006mol of acetylacetone, 0.012mol of tetrabutyl titanate into a 200ml round bottom flask, stir well at 20°C, add 0.3mol of deionized water, and the solution After turbidity, stop stirring, let stand for 45 minutes, filter, wash with absolute ethanol for 2 to 4 times, and dry at 25°C to 30°C to obtain a solid.
[0042] Step (2) Add 1 g of the solid obtained in step (1), 0.2 g of urea, add 16 ml of absolute ethanol, and 4 ml of deionized water, place it in an autoclave lined with polytetrafluoroethylene, let it stand at 130 ° C for 8 hours, and after cooling After filtering, washing with acetone and methanol for 2-3 times, drying in vacuum at high temperature for 6 hours, and sintering in a muffl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com