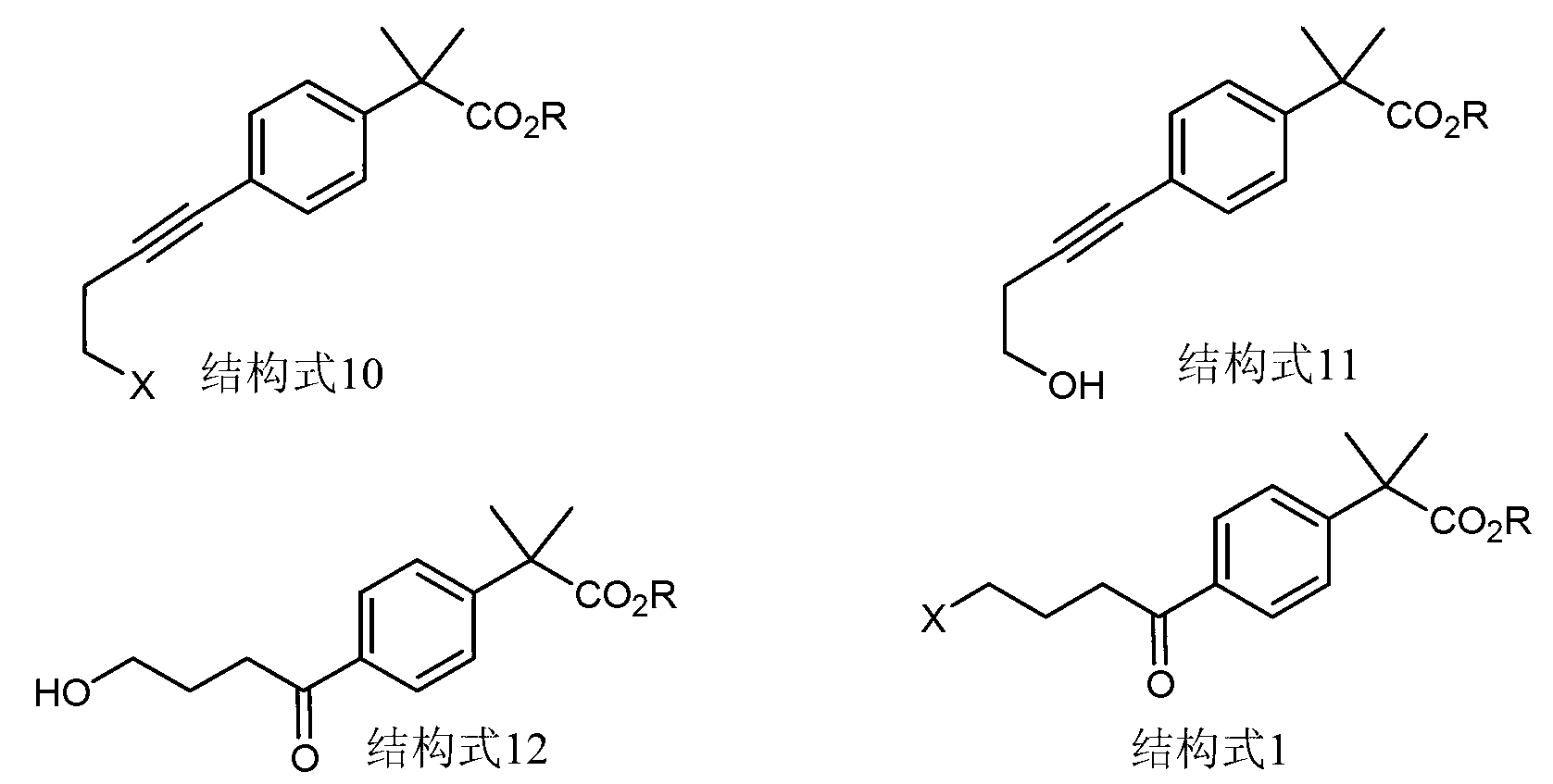
Synthetic method of 4-(4-halobutyryl)-alpha, alpha-dimethyl phenylacetate
A technology of dimethyl phenylacetate and halobutyryl, applied in the field of organic compound synthesis, can solve the problems of high cost, low yield, complicated operation and the like, and achieve the effects of low cost, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1) Synthesis of 4-chloro-1-butyne:
[0062]
[0063] In a 100ml round bottom flask, add 3-butyn-1-ol (5.4g, 77.04mmol) and 4 drops of pyridine in an ice-water bath, stir for a few minutes, then add SOCl with a constant pressure dropping funnel 2 (9.2g, 77.04mmol, added dropwise for about 15 minutes), remove the ice-water bath after the addition, and stir for 30 minutes. Then, the temperature was raised to 70°C for 3.5 hours, and the reaction was stopped. The reaction solution was poured into 150ml of ice water, extracted 3 times with dichloromethane, 50ml of dichloromethane / each time (recorded as 3×50ml), and the organic layer was NaHCO 3 Wash with saturated solution (2×100ml), and wash with water (2×100ml). Then use anhydrous Na 2 SO 4 The organic layer was dried, dichloromethane was distilled off and fractions between 70°C and 84°C were collected to obtain 2.9 g of colorless liquid, namely 4-chloro-1-butyne. Repeat the operation once or increase the amount of ...
Embodiment 2
[0073] 1) Preparation of methyl 4-(4-hydroxy-1-ynyl)-α,α-dimethylphenylacetate
[0074]
[0075] Under nitrogen protection, first add 4-bromo-α, α-dimethylphenylacetic acid methyl ester (12.8g, 49.7mmol) and triethylamine (10.06g, 99.4mmol) to a 100ml three-necked flask, then add triphenyl Phosphorus (0.156g, 0.6mmol) and cuprous iodide (0.038g, 0.3mmol) were heated up to 80°C and palladium dichloride (0.009g, 0.05mmol) was added, and kept at this temperature for 30 minutes. Then the temperature was lowered to 70°C to 73°C, and 3-butyn-1-ol (3.48g, 49.7mmol) was slowly added with a syringe pump. After about 3 hours of dropwise addition, the reaction was basically completed. 100ml of ethyl acetate was added, filtered, and the filtrate was distilled to remove the solvent to obtain 11.2g of a brownish-yellow liquid, that is, methyl 4-(4-hydroxy-1-ynyl)-α,α-dimethylphenylacetate.
[0076] 2) Preparation of methyl 4-(4-chloro-1-ynyl)-α,α-dimethylphenylacetate
[0077]
[00...
Embodiment 3
[0085] 1) Preparation of methyl 4-(4-hydroxy-1-ynyl)-α,α-dimethylphenylacetate
[0086]
[0087] Under nitrogen protection, add 4-bromo-α, α-dimethylphenylacetic acid methyl ester (12.8g, 49.7mmol) and triethylamine (10.06g, 99.4mmol) into a 100ml three-necked flask, and add triphenylphosphine (0.156g, 0.6mmol) and cuprous iodide (0.038g, 0.3mmol), warming up to 80°C, adding palladium dichloride (0.009g, 0.05mmol), keeping at this temperature for 30 minutes. Then the temperature was lowered to 70°C to 73°C, and 3-butyn-1-ol (3.48g, 49.7mmol) was slowly added with a syringe. After about 3 hours of dropwise addition, the reaction was basically completed. 100ml of ethyl acetate was added, filtered, and the filtrate was distilled to remove the solvent to obtain 11.2g of a brownish-yellow liquid, that is, methyl 4-(4-hydroxy-1-ynyl)-α,α-dimethylphenylacetate.
[0088] 2) Preparation of methyl 4-(4-hydroxybutyryl)-α,α-dimethylphenylacetate
[0089]
[0090] 4-(4-Hydroxy-1-yn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com