Method for measuring activated factor VII level in a sample
A technology for activating factors and testing samples, applied in biochemical equipment and methods, measuring devices, biological testing, etc., can solve the problems of inaccurate and difficult operation of FVIIa concentration measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1: Preparation of plasma lacking FVII
[0121]A polyclonal antibody raised in rabbits against purified human plasma FVII was linked to CNBr-activated Sepharose (Pharmacia), and 2 ml of the obtained gel was placed in a column. The column was equilibrated with 25 ml of equilibration buffer (0.15M NaCl, 10 mM citrate, pH 7.4). Then, 6 mL of human plasma was passed through the column multiple times. Under these conditions, FVII is retained immobilized on the column and the eluate (FVII-deficient plasma) is recovered. The immobilized FVII was eluted with 20 mL of regeneration buffer (50 mM NaCl; 0.1 M glycine, pH 2.4) to regenerate the column, and then the column was reconditioned with 20 mL of equilibration buffer (10 mM citrate; 0.15 M NaCl, pH 7.4). rebalance.
Embodiment 2
[0122] Example 2: Preparation of plasma lacking FVII and FVIII, FIX or FXI
[0123] A polyclonal antibody raised in rabbits against purified human plasma FVII was linked to CNBr-activated Sepharose (Pharmacia), and 2 ml of the obtained gel was placed in a column. The column was equilibrated with 25 ml of equilibration buffer (0.15M NaCl, 10 mM citrate, pH 7.4). Then, 6 mL of commercially available FVIII, FIX or FXI depleted plasma obtained from Diagnostica Stago was passed through the column several times. Under these conditions, FVII is retained immobilized on the column and the eluate (plasma lacking both FVII and FVIII, FIX or FXI) is recovered. The immobilized FVII was eluted with 20 mL of regeneration buffer (50 mM NaCl; 0.1 M glycine, pH 2.4) to regenerate the column, and then the column was reconditioned with 20 mL of equilibration buffer (10 mM citrate; 0.15 M NaCl, pH 7.4). rebalance.
Embodiment 3
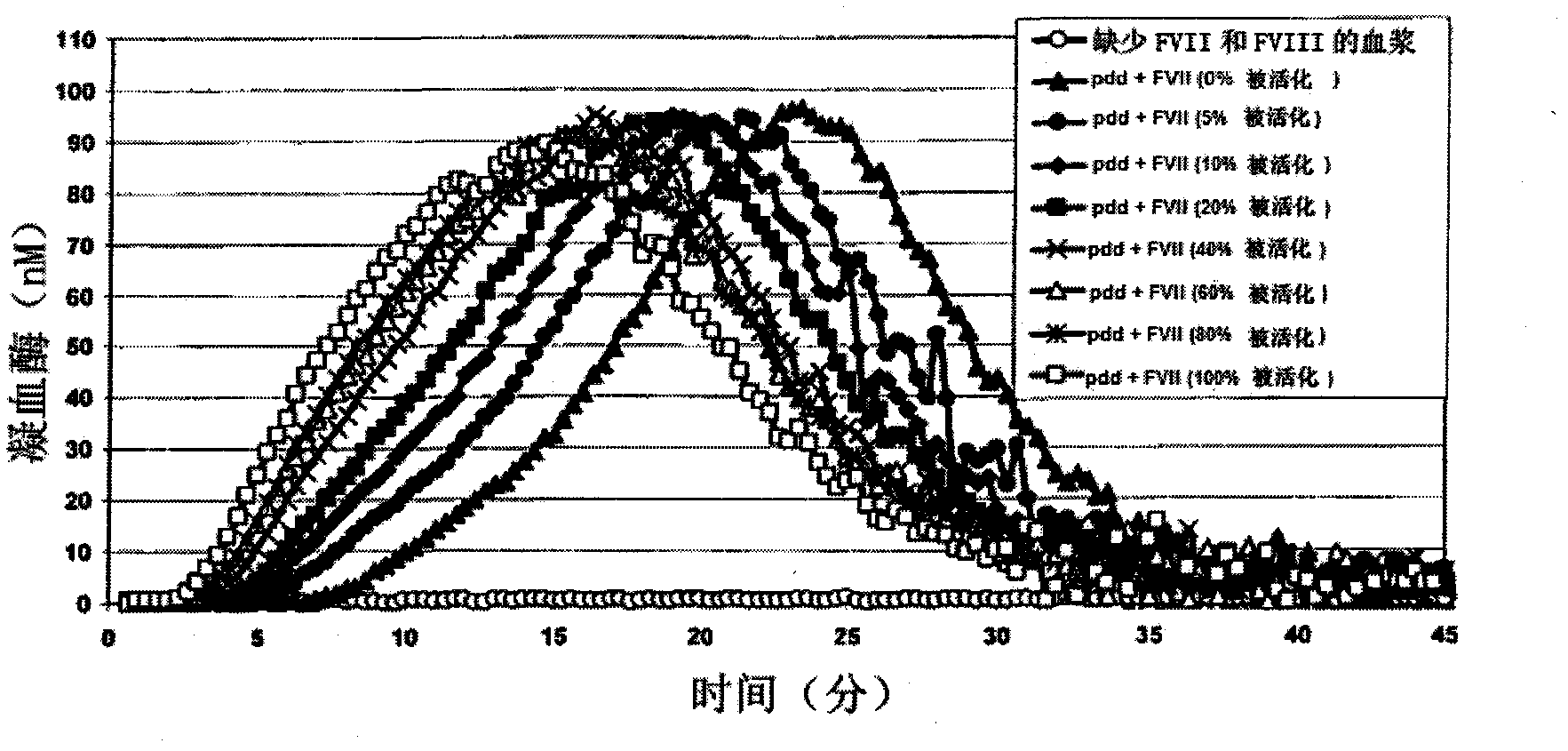
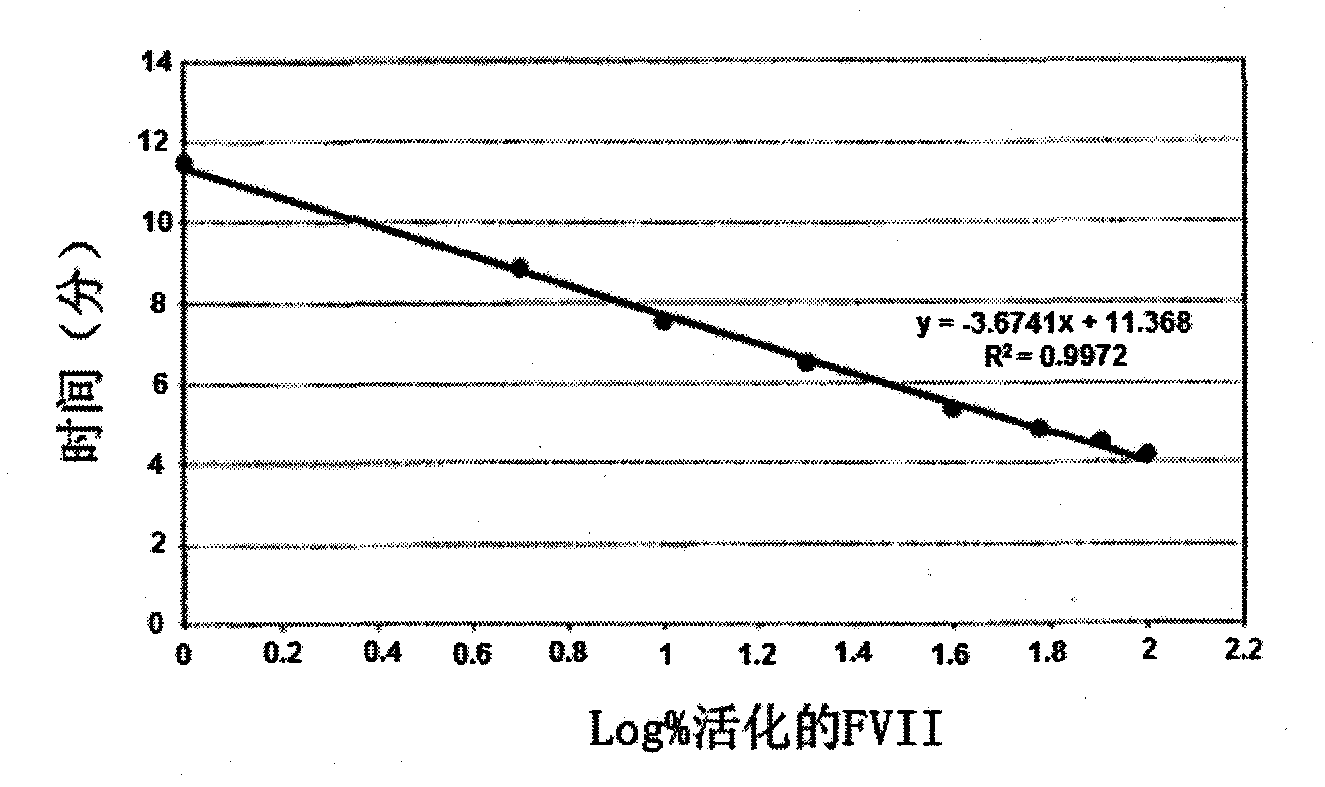
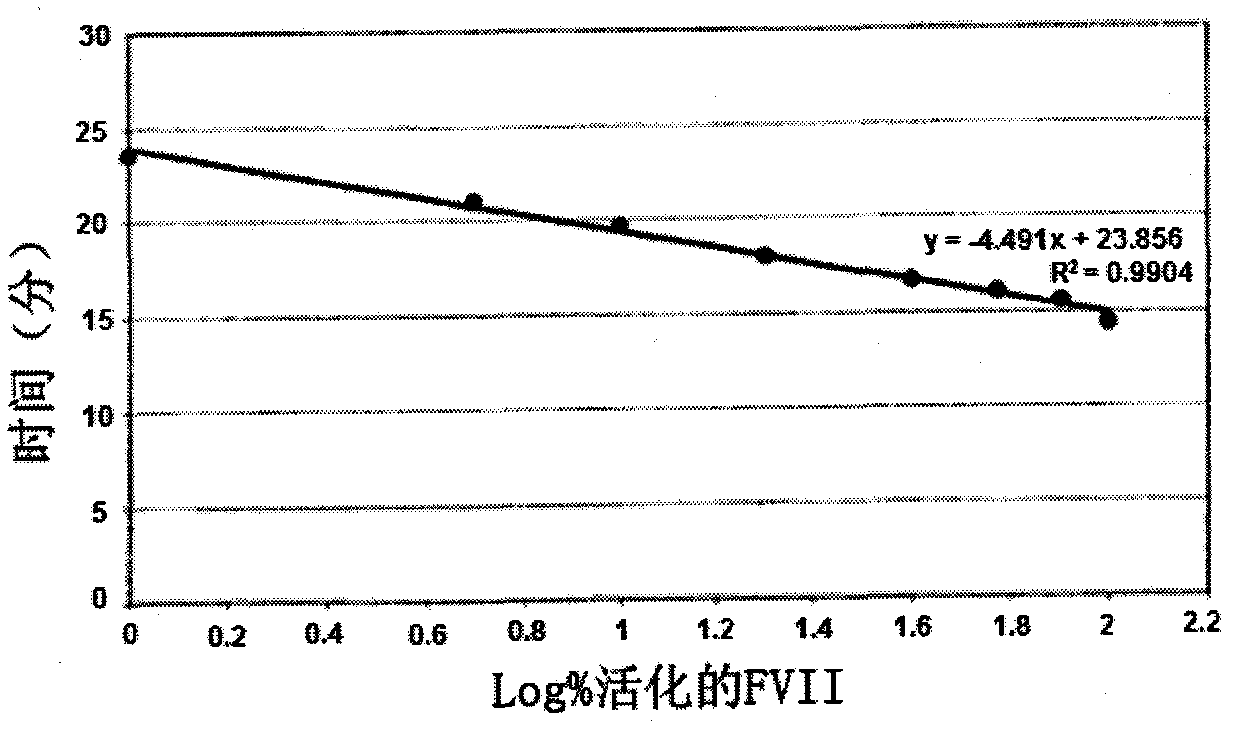
[0124] Example 3: Preparation of a standard thrombin generation profile from plasma lacking FVII and FVIII, The concentration of FVII+FVIIa is 50pM
[0125] Obtain a sufficient volume sample of International Standard FVII (SI-FVII) supplied by NIBSC (SI-FVII) and / or FVIIa (SI-FVIIa) also supplied by NIBSC, in order to obtain a standard containing a known amount of activated FVII, which is added to into 80 μL of FVII and FVIII-depleted plasma (FVIII-depleted plasma was obtained from Stargo Diagnostics, or hemophilia A plasma, which was then depleted of FVII as in Example 2), In order to obtain a mixture comprising a fixed activated FVII content between 0% and 100%, a concentration of FVII+FVIIa comprised between 10 pM and 80 pM was included. Add 20 μL of the factor that triggers the thrombin generation reaction (Ca 2+ , phospholipids and TF), at a final concentration of 5 pMTF, 1 μM phospholipids, (Stargo Diagnostics 86195 reagent diluted 1:4) and 16.7 mM Ca 2+ , and 20 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com