Fiber-reactive azo dyes, preparation thereof and use thereof
A technology of azo dyes and coupling, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems that do not fully meet this demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
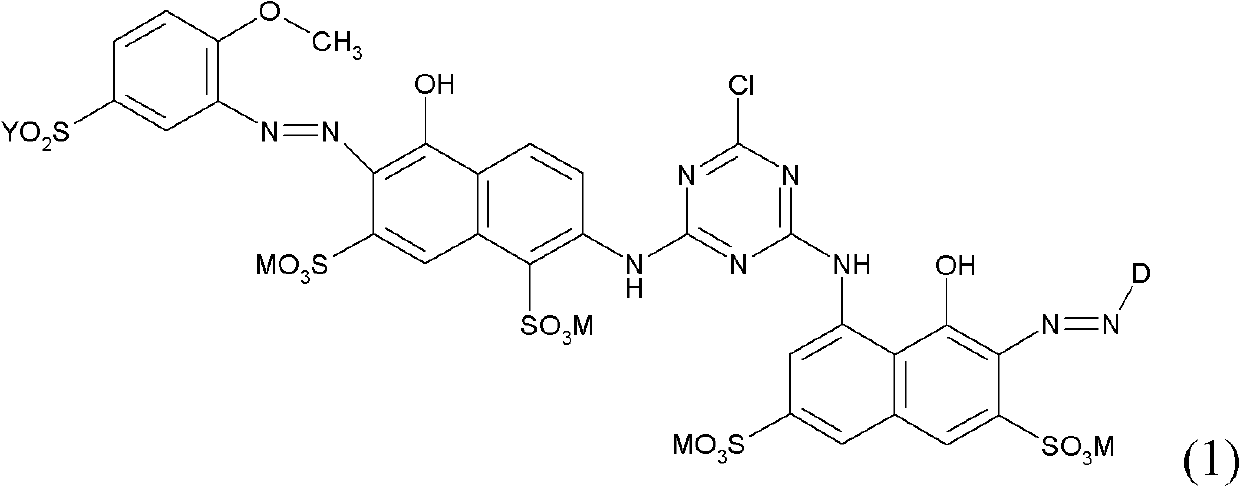
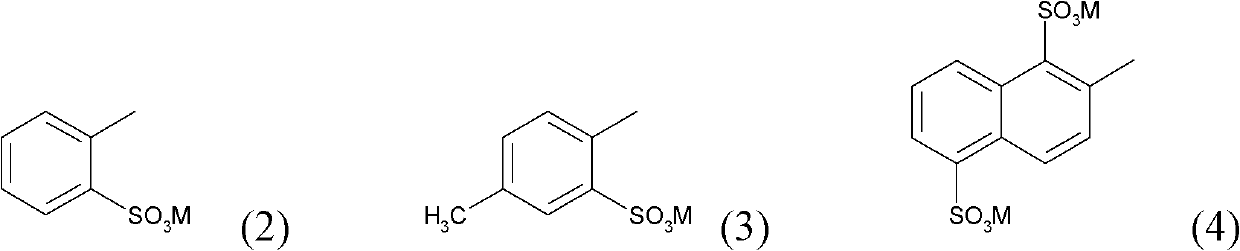
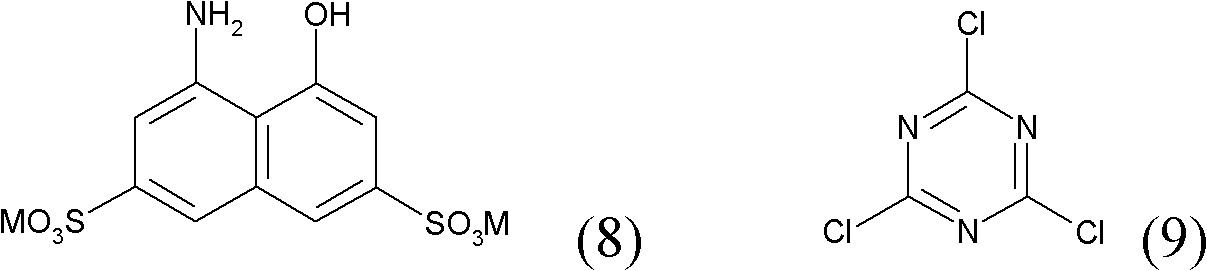
[0086] a) Suspend 19 parts of cyanuric chloride in 500 parts of water and 100 parts of ice. 32 parts of 2-amino-5-naphthol-1,7-disulfonic acid were added, followed by stirring at pH 1.5 at 0-5°C for about 3 hours.
[0087] b) 30 parts of 5-(β-sulfatoethanesulfonyl)-2-methoxyaniline are dissolved in 200 parts of water at pH 6-7. 7 parts of sodium nitrite were added, and the solution was added dropwise to a mixture of 20 parts of concentrated hydrochloric acid, 100 parts of ice and 50 parts of water. This was followed by stirring at 0-5°C for 2 hours. Decompose excess nitrite with sulfamic acid.
[0088] c) The diazo compound prepared according to b) is added dropwise to the reaction mixture obtained according to a) while maintaining the pH between 5 and 6 with 20% sodium carbonate solution. During this process the temperature was raised to room temperature.
[0089] d) Subsequently, 28 parts of 1-amino-8-naphthol-3,6-disulfonic acid are added at pH 3.5-4 and at a temperatur...
example 2
[0096] In a manner similar to that of Example 1, a dye (λ max = 518nm).
[0097]
example 3
[0099] In a manner similar to that of Example 1, a dye (λ max = 511 nm).
[0100]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com