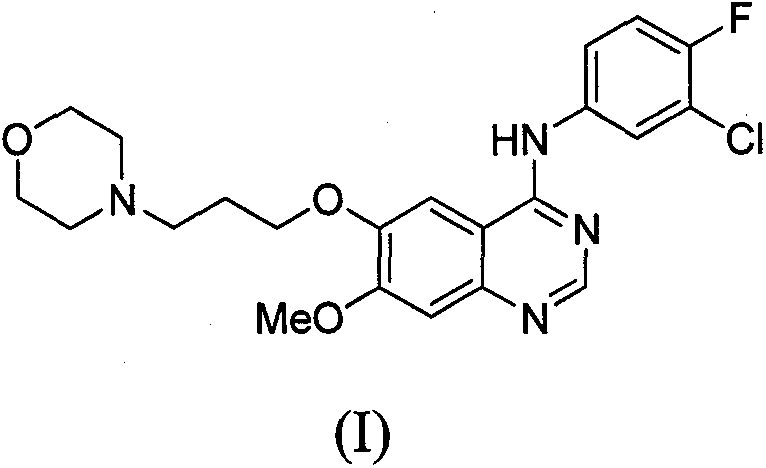
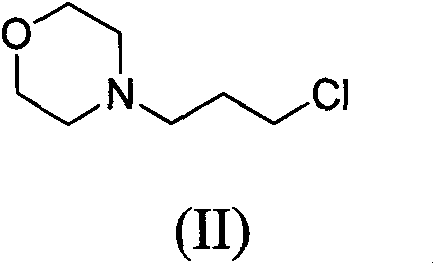
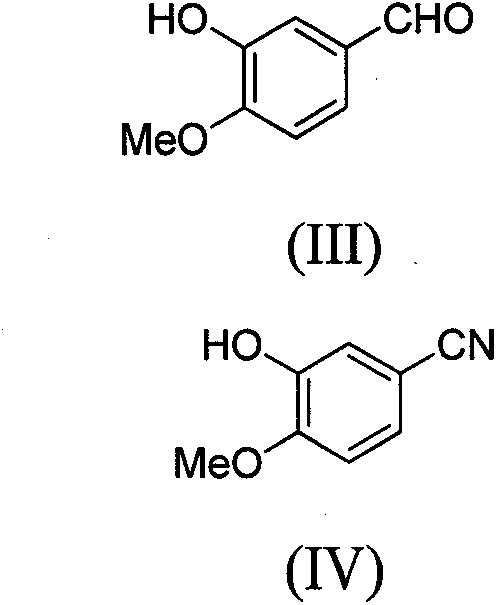
Novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline
The technology of morpholine propoxy and fluorophenyl amine group is applied in the field of preparation of 4--7-methoxy-6-quinazoline, and can solve the problems of purification of untargeted products, decreased yield, and easy raw material amidines. decomposition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The synthesis of embodiment one 3-morpholine propyl chloride (II)
[0063] Dissolve 193 g of morpholine in 580 ml of toluene and heat up to 85°C. Slowly drop 159 g of 1-bromo-3-chloropropane into the above mixture, and stir at 85°C for 1 day.
[0064] Under an ice bath, 500 ml of 18% hydrochloric acid aqueous solution was slowly added dropwise to the reaction liquid, and after stirring well, the water layer was separated. Also under ice bath, 250 ml of 50% sodium hydroxide aqueous solution was slowly added dropwise to the water layer. The aqueous layer was extracted three times with ethyl acetate and the aqueous layer was removed. After the organic layer was dried over anhydrous sodium sulfate, the organic solvent was removed under reduced pressure to obtain 147 g of product, with a yield of 90%.
Embodiment 2
[0065] The synthesis of embodiment two 2-hydroxyl-3-methoxybenzonitrile (IV)
[0066] 150 grams of 3-hydroxy-4-methoxybenzaldehyde (III), 205 grams of sodium formate were dissolved in 1.5 liters of formic acid. When the system was heated to 85° C., 97 g of hydroxylamine sulfate was slowly added to the above mixture within 6 hours, followed by reaction for 6 hours.
[0067] After cooling to room temperature, 5 liters of saturated saline was added to the reaction system, and the solid was filtered out. After drying, 145 g of white solid was obtained with a yield of 99%.
Embodiment 3
[0068] Example 3 Synthesis of 3-(3-morpholine propoxy)-4-methoxybenzonitrile (V)
[0069] 89 g of 2-hydroxy-3-methoxybenzonitrile (IV), 108 g of 3-morpholinopropyl chloride (II), 141 g of potassium carbonate were dissolved in 1 liter of DMF. After heating to 75°C, the reaction was carried out for 4 hours.
[0070] 1.5 liters of water was added to the reaction mixture, which was extracted three times with 2 liters of ethyl acetate. After the organic layer was dried over anhydrous sodium sulfate, the organic solvent was removed to obtain 165 g of viscous liquid with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com