Compositions and methods for treating influenza
A kind of influenza and composition technology, applied in the field of compositions and methods for treating influenza, and can solve problems such as long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0172] Example 1: Peptide Synthesis
[0173] All peptides described herein were synthesized by solid phase peptide synthesis (SPPS). Generally, the C-terminal amino acid is linked to a cross-linked polystyrene (or PEG-based) resin through an acid-labile bond formed with a linker molecule. The resin is insoluble in the solvents used in the synthesis, allowing for relatively simple and quick flushing of excess reagents and by-products. The N-terminus is protected with an Fmoc group which is stable in acid but removable by base. Any pendant functional groups are protected with base-stable but acid-labile groups. The synthesis is then based on the incorporation of Fmoc-protected amino acids on N-alpha into growing peptide chains, one end of which remains attached to the solid phase.
[0174] The following is an exemplary SPPS method for making the peptides described herein. To initiate each coupling, the Fmoc group on the NovaPEG resin-bound amino acid / peptide was removed usin...
example 2
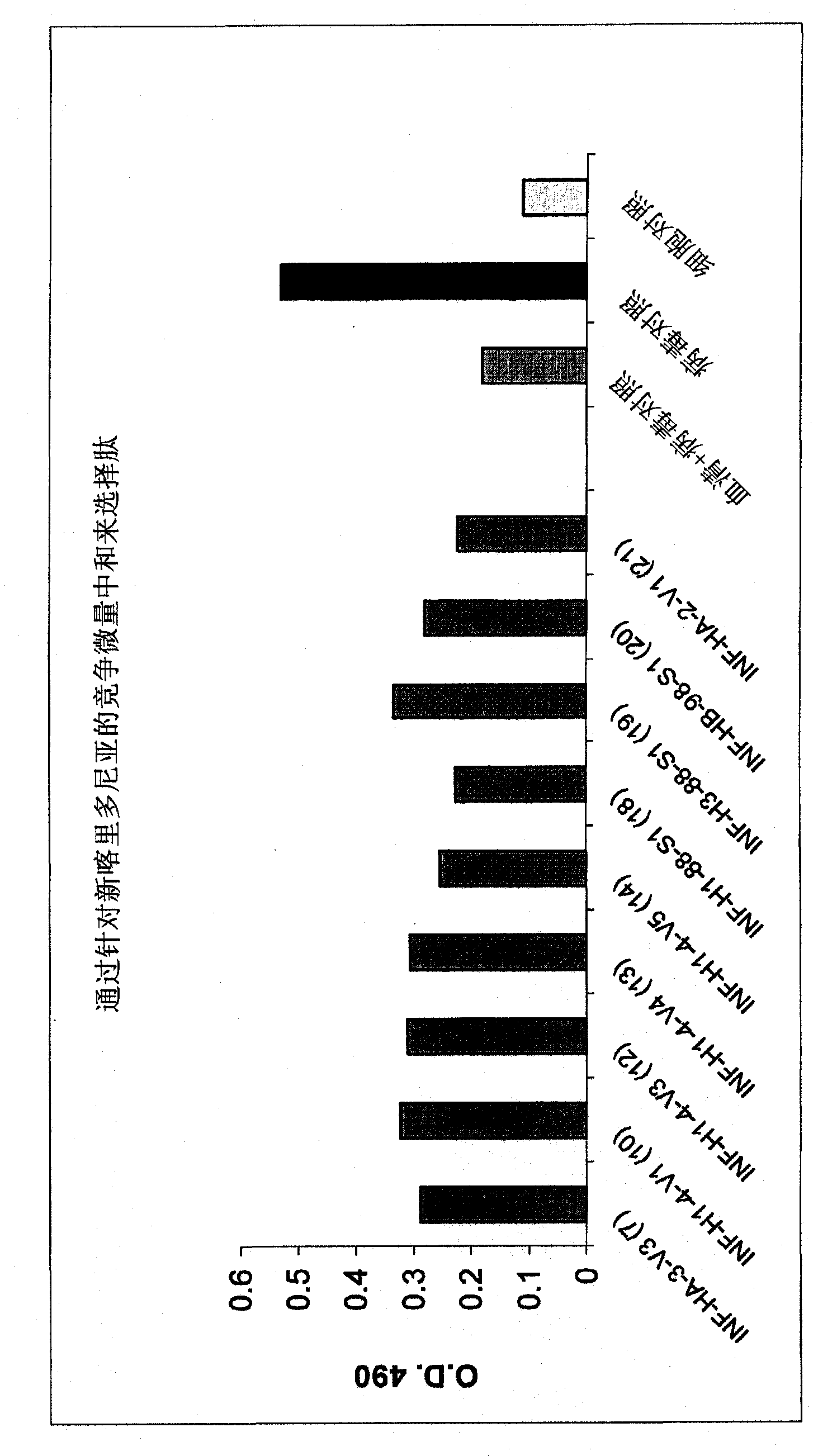
[0176] Example 2: Competitive Microneutralization Assay
[0177] In this example, various peptide compositions were evaluated for their ability to bind influenza neutralizing antibodies. Dilute different peptide compositions (see Figure 1 and Tables 1-9 below) with Iscove's Modified Dulbecco's Medium (Iscove's Modified Dulbecco's Medium, IMDM) to be 80 μg / ml, and 50 μl was added to 96 wells in separate wells in a flat bottom microtiter plate (Nunc). Each peptide composition may include all variants, as appropriate (e.g., the 8 INF-HA-3-V3 peptide variants shown in Table 1, the 4 INF-H1-4-V4 peptide variants in Table 4, etc. ). Commercially available anti-influenza sera and human sera were diluted 1 / 40 with IMDM, and 50 μl was added to each well, followed by incubation of the plate at 37° C. for 1 hour. 50 μl containing 1 x 10 5 IMDM of a pfu influenza strain Wisconsin (A / Wis / 67 / 05) or New Caledonia (A / NC / 20 / 99) was added to each well and the plate was incubated at 37°C for...
example 3
[0200] Example 3: ELISPOT analysis
[0201] In this example, the immunogenicity of the peptide composition of Example 1 was tested by measuring activation of human PBMCs (as measured by IFNγ production) in an ELISPOT assay. Multi-screen HTS plates were coated with PBS containing 10 μg / ml anti-mouse IFNγ antibody (mAb AN18, Mabtech, Mariemont, OH) at 4°C (Millipore, Bedford, MA) overnight. Plates were then washed with PBS and blocked with IMDM containing 10% FCS and 100 U / ml penicillin / streptomycin for 1 hour at room temperature. The culture medium was removed, and 2×10 5 Peripheral blood mononuclear cells (PBMC) (200 μl per well) and peptide composition (40 μg / ml, see figure 2and Table 1-9) were mixed, added to each well, and maintained for 2 days. After incubation, the cells were removed, washed with PBS+0.05% Tween 20, and incubated with 1 μg / ml biotin-labeled anti-mouse IFNγ antibody (mAb R4-6A2-biotin, Mabo Technology Co., Ltd.) for 2 hours at room temperature . Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com