Application of o-quinone structure-contained compounds in preparing tumor resistant medicament
A compound, o-quinone technology, applied in the field of anti-tumor compounds, can solve the problems of low content, unsatisfactory antibacterial and anti-tumor activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] 1. Preparation of the series of compounds involved in the present invention.
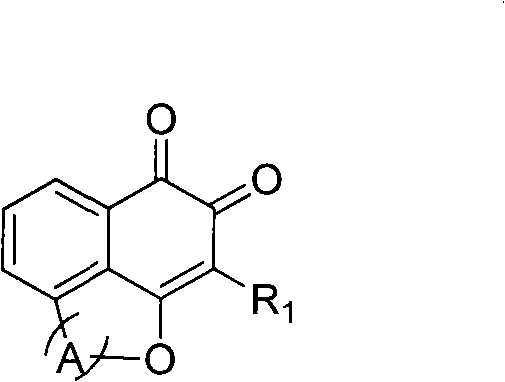
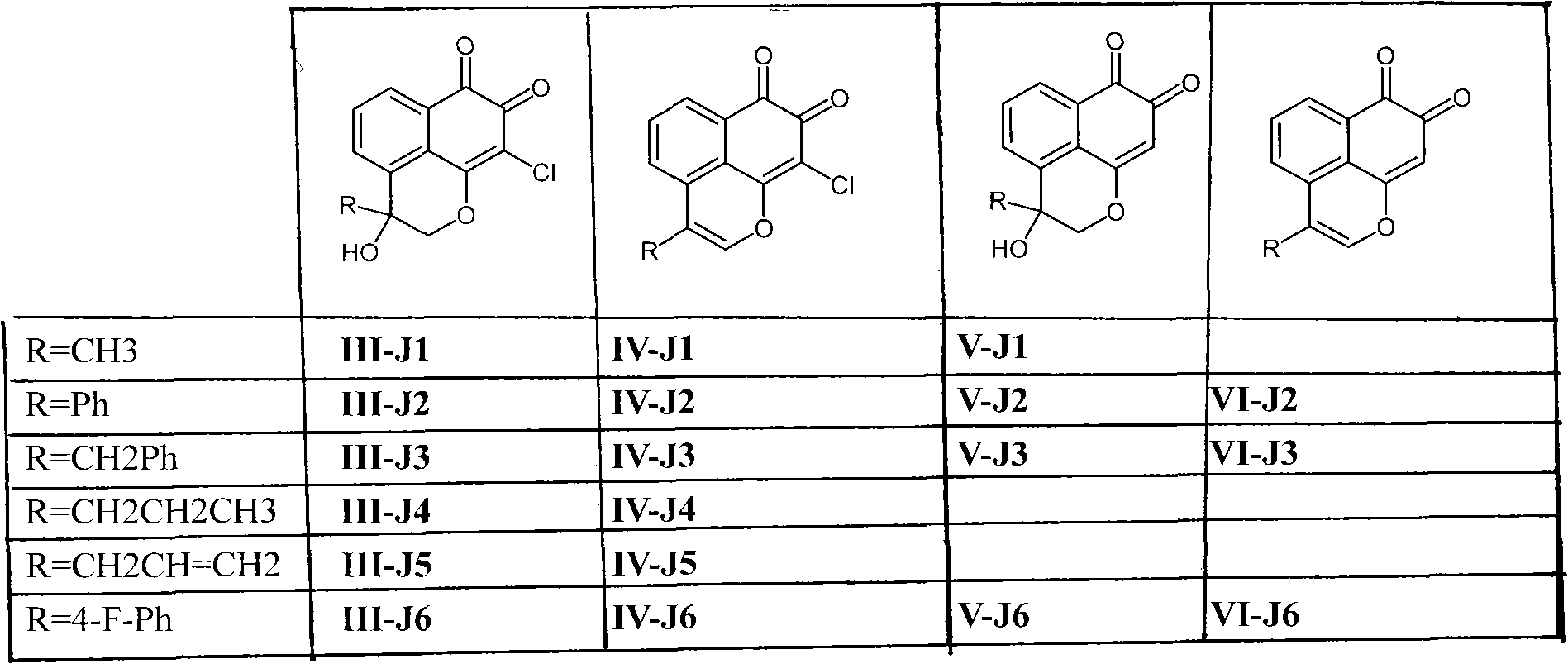
[0021] The preparation method of the compound represented by general formula (I) involved in the present invention can be carried out with full reference to existing patents 200410077779.3, 200410077778.9 and WO 01 / 51004A2. According to the patent, we have synthesized compounds III-J1~6, V-J1~ 3. V-J6, VII-J1, VIII-J1, IV-J2~6, VI-J2, VI-J3, VI-J6 compounds, the compound spectrum data obtained are the same as the above patent results, and will not be listed here out.
[0022] Compound structures and corresponding numbers are as follows:
[0023]
[0024] VIII-J1: R1=Cl, R2=CH3, R3=OCOPh VII-J1: R1=H, R2=CH3, R3=H
[0025]
[0026] D-1 D-2 D-3 D-4 D-5
[0027] 2. Cytotoxic activity test experiment
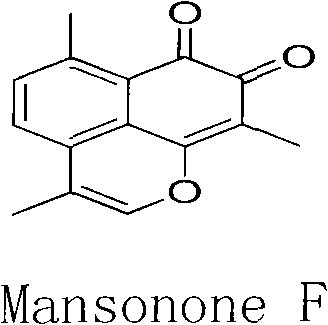
[0028] Cytotoxic activity of the synthesized compounds III-J1~6, V-J1~3, V-J6, VII-J1, VIII-J1, IV-J2~6, VI-J2, VI-J3, VI-J6 Test, with 10-hydroxycamptothecin, mansononeF, etoposide an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com