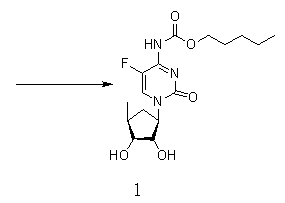
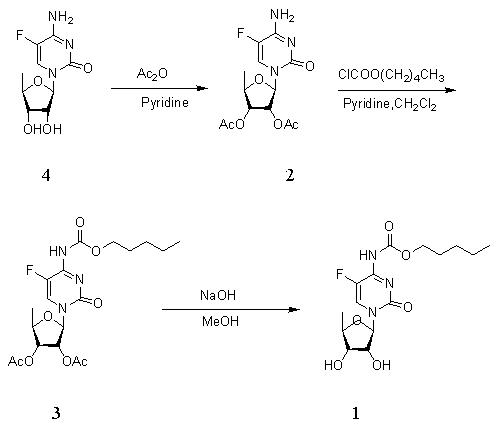
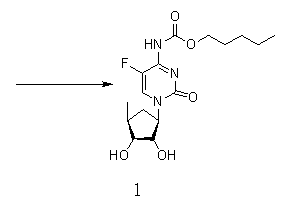
Novel synthesis process of capecitabine
A technology of capecitabine and synthesis process, which is applied in the direction of organic compound/hydride/coordination complex catalyst, preparation of sugar derivatives, sugar derivatives, etc. It can solve the problems of heavy metal pollution and high cost, and achieve total revenue High efficiency, simple post-treatment, and the effect of avoiding heavy metal pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific embodiments.
[0034]
[0035]
[0036]
[0037] Synthesis of Compound II:
[0038] 134 grams of compound Ⅰ (1.00mol) was added to 800 milliliters of N,N-dimethylformamide, under stirring, 8.6 grams of p-toluenesulfonic acid (0.05mol) was added, and 124.8 grams of 2,2-dimethoxy Propane (1.20mol), stirred at room temperature overnight, after the reaction, concentrated under reduced pressure to distill unreacted 2,2-dimethoxypropane and N,N-dimethoxypropane, the residue was added to 500ml of water, Use 10% sodium carbonate aqueous solution to adjust PH=7-8, add 1200 ml of ethyl acetate for extraction, the ethyl acetate layer is washed with saturated brine, and dried over anhydrous sodium sulfate; filter, and the filtrate is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com