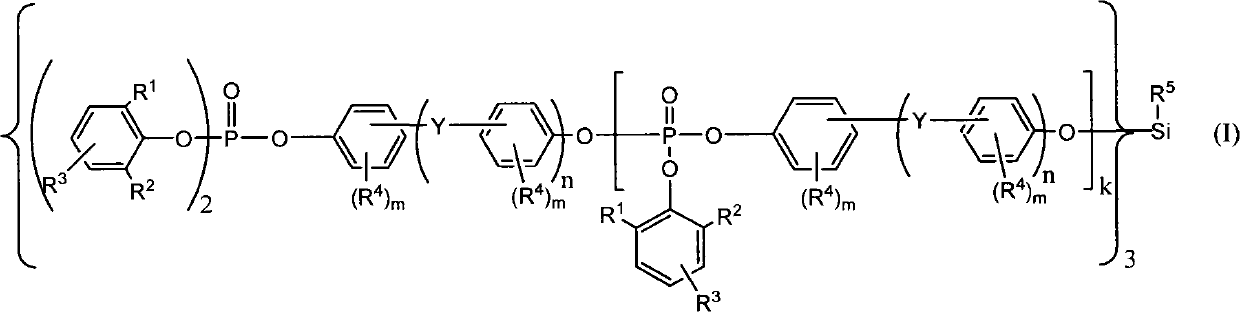
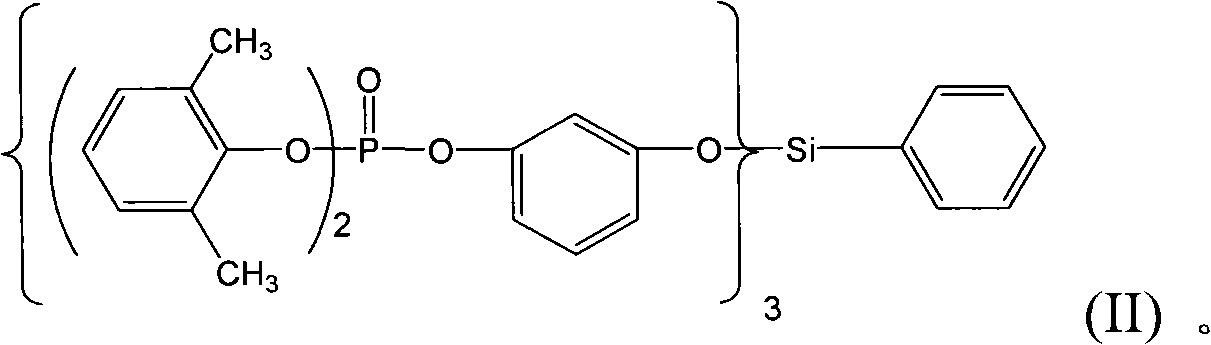
Organic silicon phosphate and preparation method thereof
A technology of organosilicon phosphate and alkyl, which is applied in the field of organosilicon phosphate and its preparation, and can solve the problems of low molecular weight, high composition, pollution, and easy volatility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
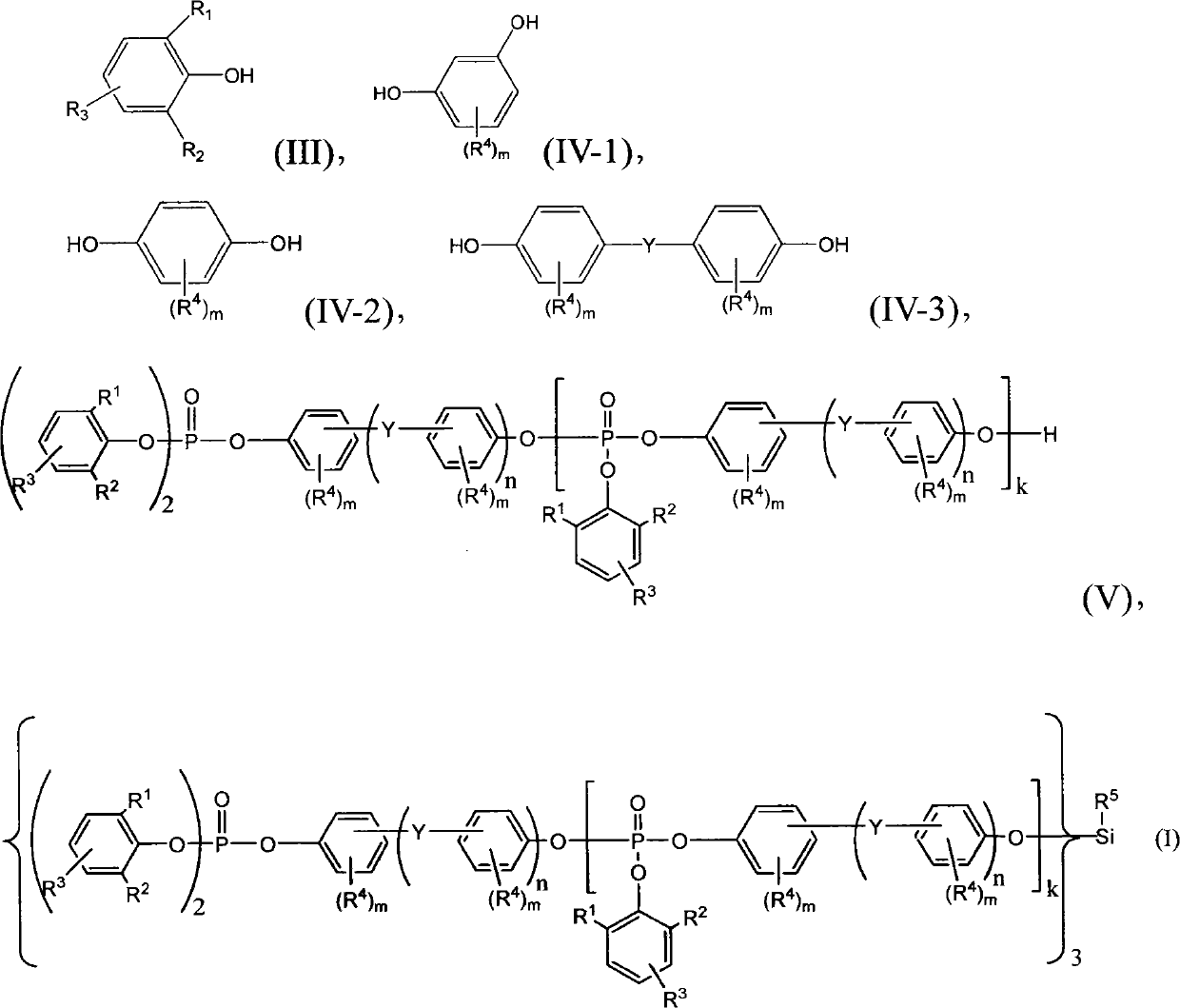
[0032] The present invention also provides a method for preparing organosilicon phosphate, which includes the following steps (a) to (c). First carry out step (a), the phenol (phenol) of formula (III) and phosphorus oxychloride (phosphoryl chloride, POCl 3 ) reacts with the ratio of molar ratio 2: (1-1.2), and its reaction formula is as follows:
[0033]
[0034] (III) Intermediate 1 Intermediate 2
[0035] where R 1 and R 2 for the same or different C 1 ~C 5 Alkyl (alkyl); R 3 for hydrogen or C 1 ~C5 Alkyl (alkyl).
[0036] After the reaction, intermediate product 1 and intermediate product 2 can be obtained, wherein intermediate product 1 is the main product, accounting for 80%-90% of the product. Hydrogen chloride (HCl) gas generated during the reaction process can be recycled through water under normal pressure or reduced pressure.
[0037] The above-mentioned phenols include dimethylphenol (dimethylphenol), diethylphenol (diethylphenol), 2-methyl-3-ethylphenol...
Embodiment 1
[0060] In a 1000mL four-necked flask equipped with a mechanical stirrer, a thermometer, a condensation tube connected to a hydrogen chloride gas absorption device, and a dropping funnel, add 244g (2.00mol) of 2,6-dimethylphenol (2,6-dimethylphenol), 7.03g (0.07mol) anhydrous magnesium chloride (anhydrous magnesiumchloride), 160g (1.04mol) phosphorus oxychloride (phosphoryl chloride, POCl 3 ) and 10g of xylene (xylene) were mixed and heated. Gradually raise the temperature to 110°C to generate a large amount of hydrogen chloride gas, react at 110°C for 2 hours, then raise the temperature to 130°C for 2 hours, then raise the temperature to 155°C for 6 hours, and then reduce the pressure to 150mmHg for 4 hours.
[0061] After the reaction mixture was cooled to 100°C, 61g (0.55mol) of resorcinol (m-bisphenol), 1.5g (0.01mol) of anhydrous aluminum chloride (anhydrous aluminum chloride) and 10g of xylene (xylene) were added, and the temperature was raised After reacting at 120°C fo...
Embodiment 2
[0064] In a 1000mL four-neck flask equipped with a mechanical stirrer, a thermometer, a condenser tube connected to a hydrogen chloride gas absorption device, and a dropping funnel, add 244g (2.00mol) of 2,6-dimethylphenol, 7.05g (0.07mol) of Magnesium chloride hydrate, 160g (1.04mol) phosphorus oxychloride (phosphoryl chloride, POCl 3 ) and 10g of xylene (xylene) were mixed and heated. Gradually raise the temperature to 110°C to generate a large amount of hydrogen chloride gas, react at 110°C for 2 hours, then raise the temperature to 130°C for 2 hours, then raise the temperature to 155°C for 6 hours, and then reduce the pressure to 150mmHg for 4 hours.
[0065] After the reaction mixture was cooled to 100°C, 110g (1.00mol) of resorcinol (m-bisphenol), 3.3g (0.02mol) of anhydrous aluminum chloride and 10g of xylene (xylene) were added, and the temperature was raised to 120°C for reaction 12 After one hour, add 70g (0.33mol) of phenyltrichlorosilane (phenyltrichlorosilane) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com