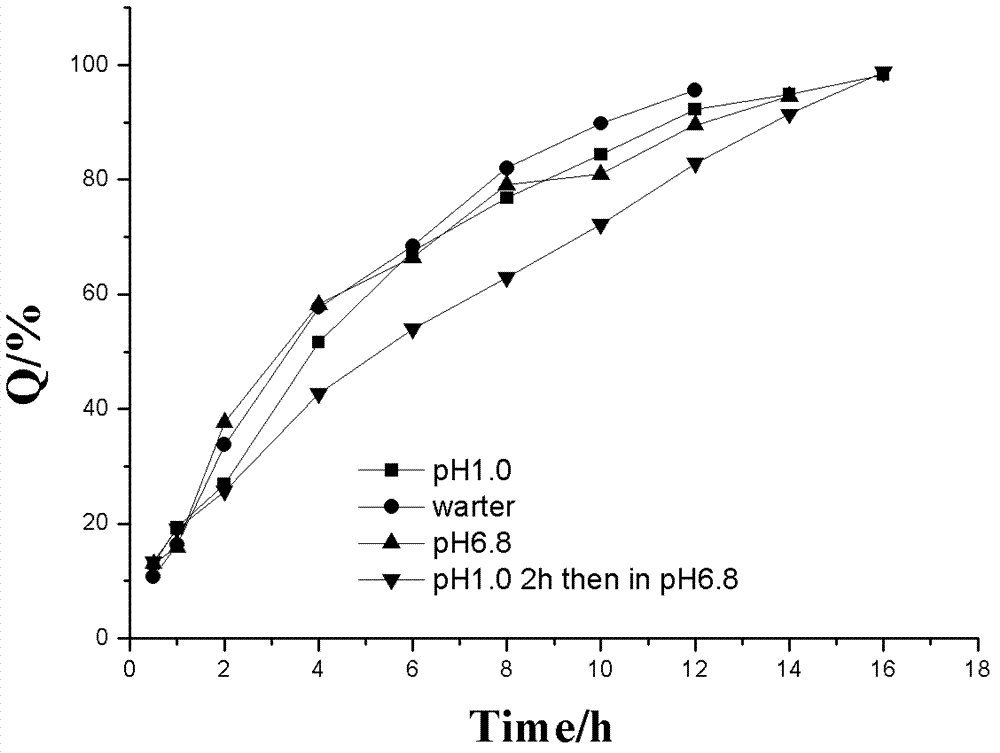
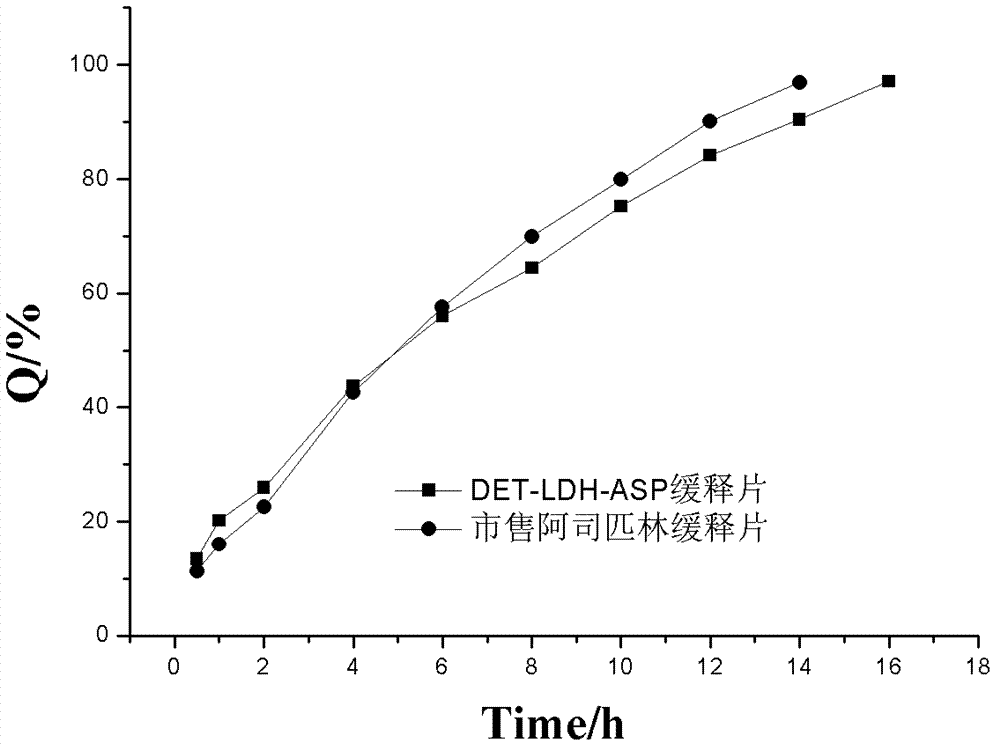
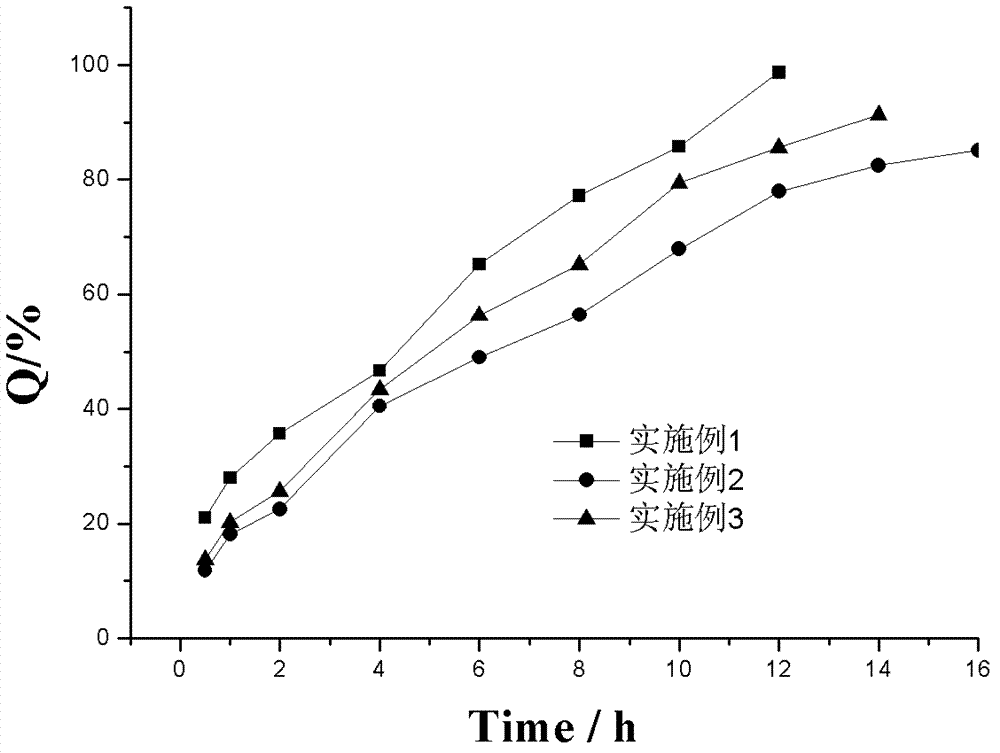
LDH (Layered Double Hydroxides) supermolecular assembly type aspirin sustained release tablet and preparation method thereof
An aspirin and supramolecular technology, applied in the field of LDH supramolecular assembled aspirin sustained-release tablets and preparation, can solve problems such as disintegration, LDH easy to erode, and failure to achieve sustained-release effects, etc., to improve sustained-release performance, transport and The release process is particularly durable and the effect of improving biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: The prescription of "DET-LDH-ASP" supramolecular sustained release tablet is as follows
[0027]
[0028] The method for preparing the DET-LDH-ASP drug-carrying system is as follows: 40.82g MgCl 2 ·6H 2 O, 24.30g AlCl 3 ·6H 2 O and 72.83g ASP are dropped into the reactor of 2000mL three-neck bottle assembly respectively, add 800mL water and dissolve to clarification, in N 2 Add 2.0mol L dropwise under the conditions of protection, 80°C constant temperature and 300rpm magnetic stirring -1 NaOH solution, control the pH of the co-precipitation end point to be 10.06, and make the LDH-ASP secondary complex slurry; add 200 mL of dextran aqueous solution with a mass concentration of 16% after in-situ crystallization for 45 min, and keep the N 2 Protection, 80°C constant temperature and 300rpm stirring conditions remain unchanged, and the in-situ reaction is performed for 40 minutes to make a DET-LDH-ASP tertiary compound slurry; transfer the prepared hot slur...
Embodiment 2
[0030] Example 2: The preparation prescription of "DET-LDH-ASP" supramolecular sustained-release tablets is as follows
[0031]
[0032] The method for preparing the DET-LDH-ASP supramolecular drug carrier is the same as that in Example 1. Tablet preparation method: weigh a certain amount of "DET-LDH-ASP" powder (80 mesh), hypromellose (80 mesh), and sodium alginate (80 mesh) according to the prescription ratio, mix the three fully, and pour A small amount of talc powder (100 mesh) is added to the mixed raw materials, mixed evenly, and the tablet is directly compressed using a die with a diameter of 8 mm to obtain a sustained-release tablet with a hardness of about 4.5 kg and a tablet weight of 0.12 to 0.16 g.
Embodiment 3
[0033] Example 3: "DET-LDH-ASP" supramolecular sustained-release tablets were prepared according to the following prescription
[0034]
[0035]
[0036] The method for preparing the "DET-LDH-ASP" supramolecular drug carrier is the same as in Examples 1-2. Tablet preparation method: Grind raw materials such as DET-LDH-ASP, hydroxypropylmethylcellulose, sodium alginate and talcum powder into powder respectively, pass the DET-LDH-ASP powder through a 80-mesh sieve, and hydroxypropylmethylcellulose Pass through an 80-mesh sieve, pass through a 80-mesh sieve for sodium alginate, pass through a 100-mesh sieve for talcum powder, and grind until it can be completely sieved; mix the powder raw materials according to the prescription ratio, grind and disperse evenly with a mortar, and then use a shallow concave with a diameter of 8mm The tablet can be directly compressed by the die to obtain a sustained-release tablet with a diameter of 8 mm, a hardness of about 4.5 kg, and a tab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com