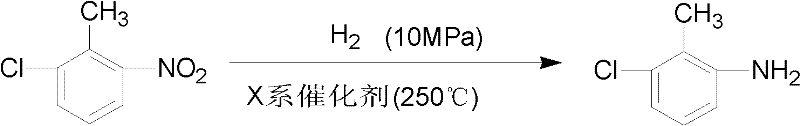
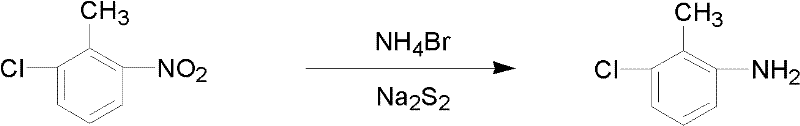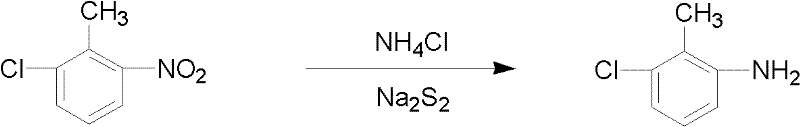Synthetic method of 3-chloro-2-methylaniline
A synthesis method, the technology of methylaniline, applied in the field of synthesis of 3-chloro-2-methylaniline, can solve the problems of catalyst poisoning, high product cost, poor activation performance, etc., and achieve low production cost and high safety , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In a 1000 ml three-neck flask equipped with mechanical stirring, reflux condenser, and thermometer, add 50 ml of water and 1 mol of sodium polysulfide to the flask, stir the mixture and add 0.4 mol of ammonium bromide, and gradually add 1 mol of 6- Chloro-2-nitrotoluene, the temperature is controlled at 30°C, and the following chemical reactions occur in the system:
[0015]
[0016] After the reaction was completed, the upper organic phase was separated, washed with water until neutral, and the organic phase was distilled at a vacuum of 0.1 MPa to collect 139.8 grams of fractions at 127-137°C.
[0017] With 3-chloro-2-methylaniline as standard, gas chromatography analysis. The analysis results are shown in Table 1 and Table 2.
[0018] Table 1.3-Chloro-2-methylaniline standard sample chromatographic analysis results
[0019] peak number
[0020] Table 2. Fraction chromatographic analysis results
[0021] peak number
[0022] The purity of 3-ch...
Embodiment 2
[0024] In a 1000ml three-neck flask equipped with mechanical stirring, reflux condenser, and thermometer, add 50ml of water and 2mol sodium polysulfide into the flask, stir the mixture system and then add 0.5mol ammonium chloride, and gradually add 1mol 6-chlorine -2-Nitrotoluene, the temperature is controlled at 105°C, and the following chemical reactions occur in the system:
[0025]
[0026] After the reaction is completed, the unreacted acid liquid in the kettle is first recovered, the upper organic phase is separated, washed with water until neutral, the organic matter is distilled, the vacuum degree is 0.1 MPa, and 140.2 grams of fractions at 127-137 ° C are collected.
[0027] With 3-chloro-2-methylaniline as standard, gas chromatography analysis. The analysis results are shown in Table 3 and Table 4.
[0028] Table 3.3-Chloro-2-methylaniline standard sample chromatographic analysis results
[0029] peak number
[0030] Table 4. Fraction chromatographic a...
Embodiment 3
[0034] In a 1000ml three-neck flask equipped with mechanical stirring, reflux condenser, and thermometer, add 50ml of water and 1mol sodium polysulfide into the flask, stir the mixture system and then add 0.6mol ammonium bisulfate, and gradually add 2mol 6-chlorine -2-Nitrotoluene, the temperature is controlled at 70°C, and the following chemical reactions occur in the system:
[0035]
[0036] After the reaction is completed, the unreacted acid liquid in the kettle is first recovered, the upper organic phase is separated, washed with water until neutral, the organic matter is distilled, the vacuum degree is 0.1 MPa, and 281 grams of fractions at 127-137 ° C are collected.
[0037] With 3-chloro-2-methylaniline as standard, gas chromatography analysis. The analysis results are shown in Table 5 and Table 6.
[0038] Table 5.3-Chloro-2-methylaniline standard sample chromatographic analysis results
[0039] peak number
[0040] Table 6. Fraction chromatographic ana...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com