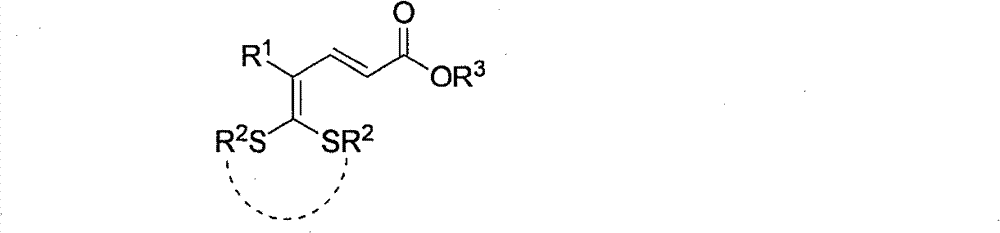
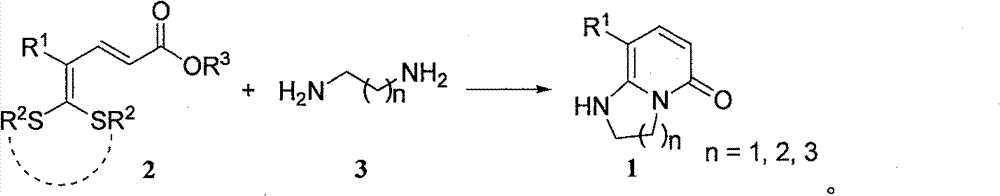
Synthetic method of bicyclic pyridone derivative
A technique for the synthesis of bicyclic pyridone and its synthesis method, which is applied in the field of synthesizing bicyclic pyridone derivatives, can solve the problems of limiting the application of bicyclic pyridone derivatives, molecular structure restrictions, etc., and achieve high product yield, simple operation, and easy reaction. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] In a 25mL Schlenk reaction flask, add 5,5-dialkylthio-2,4-pentadienoate 2a (65mg, 0.25mmol), organic diprimary amine 1,2-ethylenediamine (3a) ( 18mg, 0.30mmol) and 2mL solvent ethanol, stirred and refluxed for 14h. After the reaction, the mixture was cooled to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography, the eluent was petroleum ether (60-90°C) / acetone, (v / v=3:1) , to obtain the target product 1a (35 mg, yield 79%) as a white solid. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0032] The reaction steps and operations are the same as in Example 1, except that the reaction time is 24 hours. The reaction was stopped, and the target product 1a (32 mg, yield 72%) was obtained after post-processing. It shows that prolonging the reaction time leads to partial decomposition of the product.
Embodiment 3
[0034] The reaction steps and operations are the same as in Example 1, except that the reaction temperature is room temperature 20° C. and the reaction time is 24 hours. The reaction was stopped, and the target product 1a (8 mg, yield 17%) was obtained after post-processing. It shows that the reaction temperature is too low to slow down the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com