Quzhazhigan crystal and preparation method and application thereof
A technology for tristilbene and crystals, applied in the field of tristilbene crystals and their preparation, can solve the problems affecting drug quality, safety and effectiveness, affecting drug stability, solubility and bioavailability, affecting drug processing and Production and other problems, to achieve the effect of high solubility and bioavailability, single crystal form, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: X-ray powder diffraction, infrared spectroscopic analysis and differential scanning calorimetry analysis conditions
[0025] X-ray powder diffraction analysis: the detection instrument is D / max-3A X-ray diffractometer; the detection conditions are Cu target Kα1 ray, voltage 35kV, current 25mA, divergence slit 1°, anti-scatter slit 1°, receiving slit The slits are 0.3mm and 0.3mm, and the 2θ range is 3° to 60°; the detection basis is the general rules of X-ray diffraction methods for polycrystals with rotating targets JY / T009-1996.
[0026] Infrared spectroscopy (IR) analysis: the detection instrument is a Perkin Elmer FT-IR spectrophotometer; the detection condition is KBr pellets.
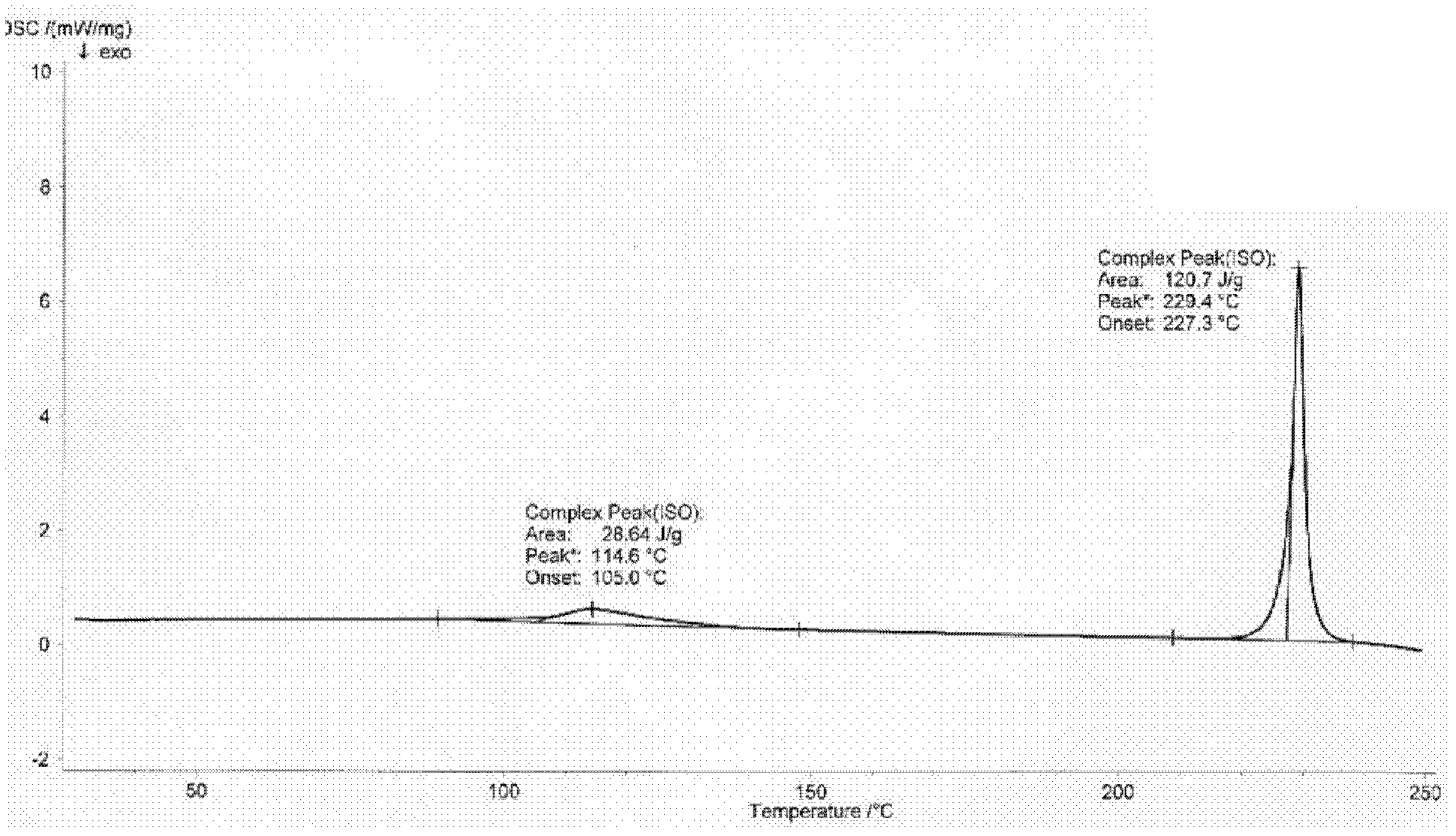
[0027] Differential scanning calorimetry (DSC) analysis: The detection instrument is DSC204 differential scanning calorimeter from NETZSCH, Germany; the detection condition is N2 as the atmosphere, 20mL / min, the temperature is raised from room temperature to 250°C at 10°C / min,...
Embodiment 2
[0028] Embodiment 2: Preparation of Trizaperoside crystals
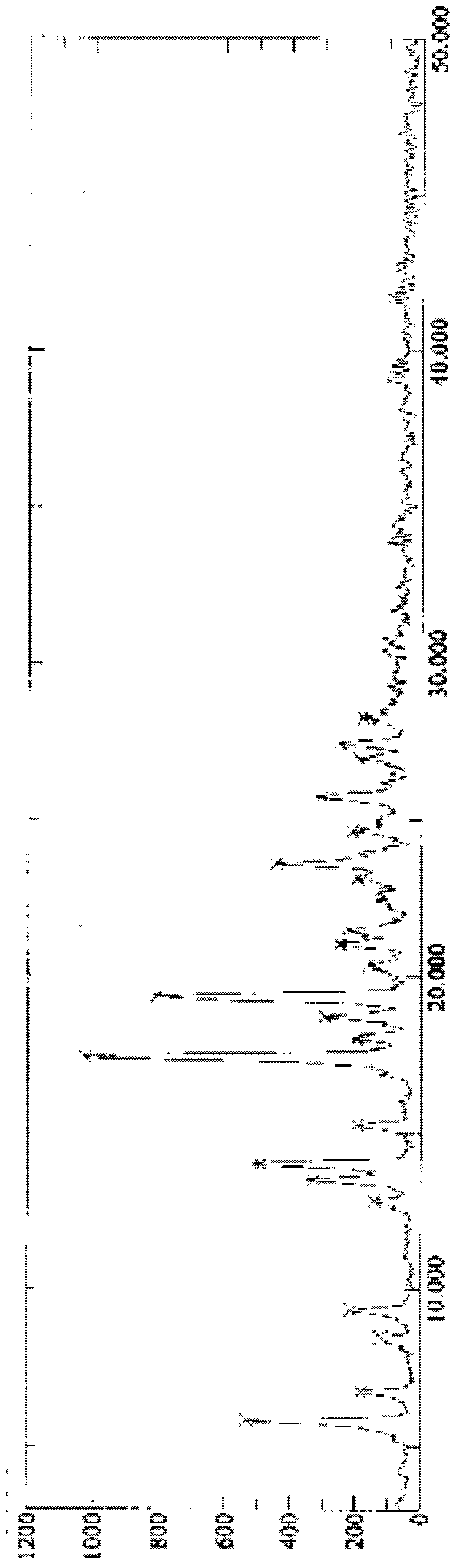
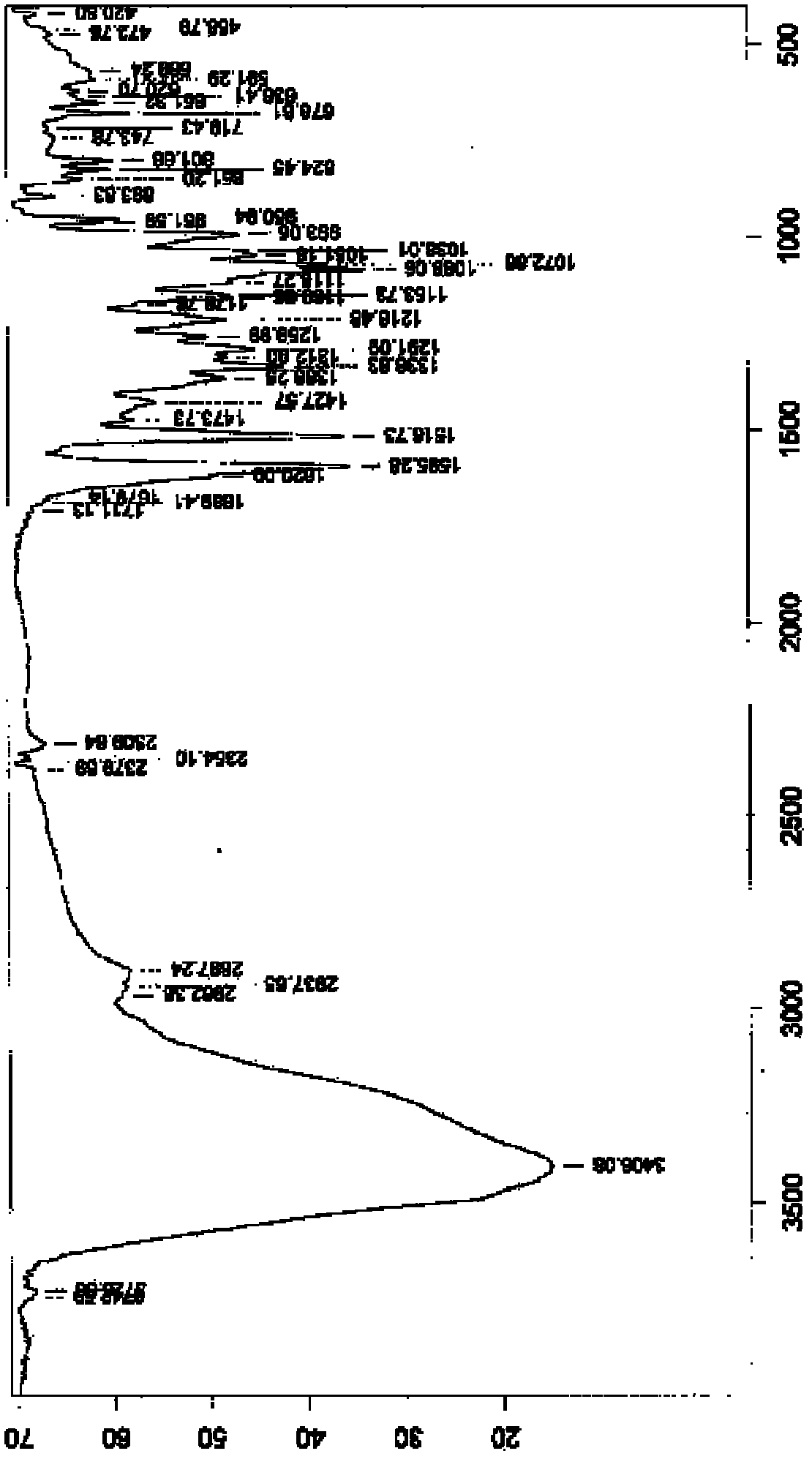
[0029] Take P 2 o 5 Add 120mL of water, then add 1.5% (that is, 0.3g) needle active boiled for 3min, filter while hot, stand at 4°C for 6h, and pump out the crystallization solution Filter, P 2 o 5 The desiccant was dried under reduced pressure for 12 hours to obtain 17.1 g of tristilbene crystals, and the yield of tristilbene crystals was 85.5%. Carry out X-ray powder diffraction, infrared spectrum analysis and differential scanning calorimetry analysis to the obtained trizapereside crystal, the results are shown in Figure 1~3 . Wherein, the calculation formula of the tristilbeside crystal yield is: tristilbene crystal yield=tristilbene crystal amount / tristilbene input amount×100%.
[0030] Depend on figure 1It can be seen that the X-ray powder diffraction pattern of the tristilbene crystal prepared in the present invention is 5.8, 6.7, 8.4, 9.3, 12.8, 13.5, 14.0, 15.2, 17.4, 18.7, 19.4, 23.6, 25.7, 27.0, 27...
Embodiment 3
[0032] Take P 2 o 5 As a desiccant, add 120 mL of the solvent listed in Table 1 to 20 g of tristilbene raw material (purity 98%) after vacuum drying for 12 hours, then add 1.5% (ie 0.3 g) of the active needle and boil for 3 minutes. Filter, stand at 4°C for 6h, suction filter the crystallization solution, P 2 o 5 The desiccant was dried under vacuum and reduced pressure for 12 hours to obtain tristilbene crystals, the yield and yield of tristilbene crystals were calculated, and the obtained tristilbene crystals were analyzed by differential scanning calorimetry and X-ray Diffraction and infrared spectroscopic analysis, the results are shown in Table 1. X-ray diffraction and infrared spectroscopic analysis are consistent with the results of Example 2, not shown here.
[0033] Table 1 Solvent Selection
[0034]
[0035] As can be seen from the results in Table 1, when using 15% ethanol, 30% ethanol, 45% ethanol, 15% methyl alcohol, 30% methyl alcohol, 45% methyl alcohol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com