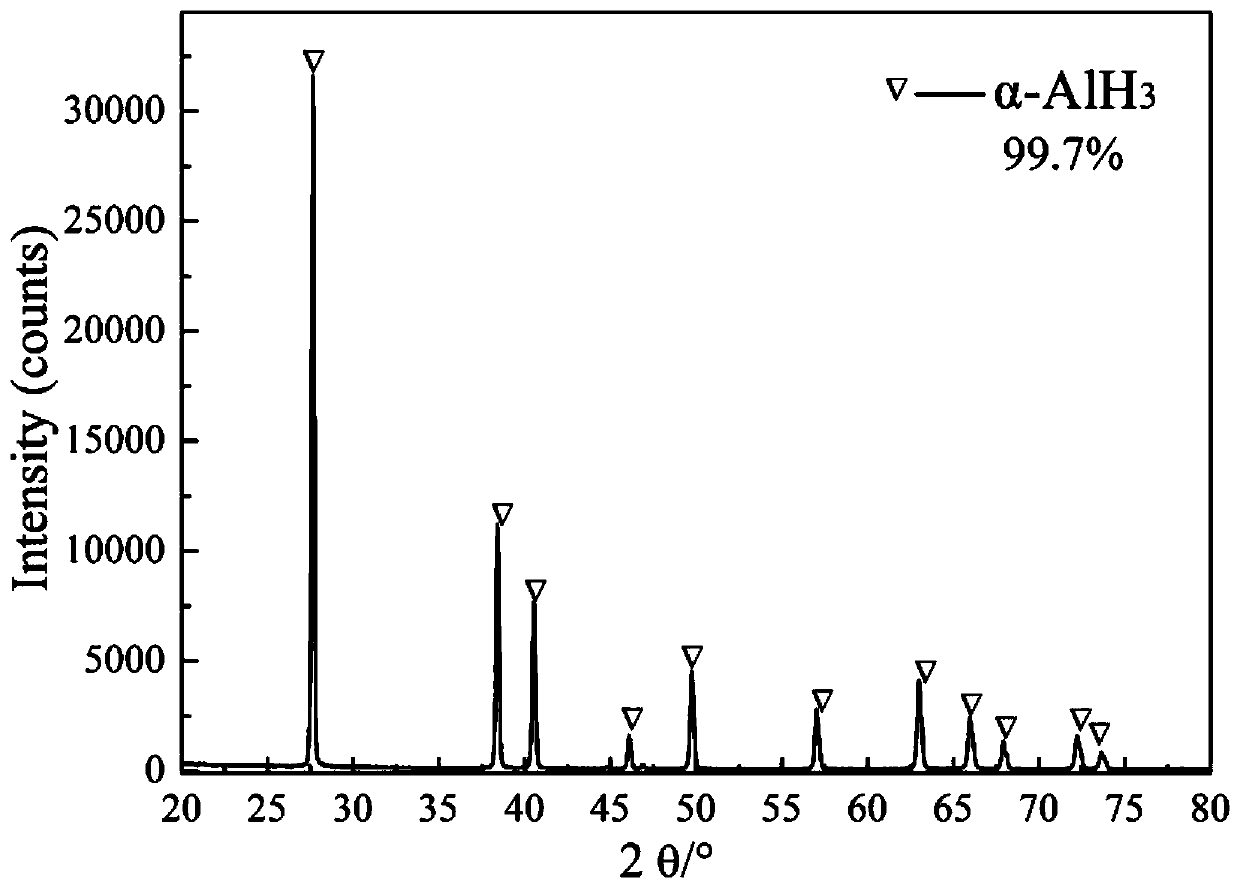
Method for preparing α-aluminum hydride by mixed catalysis of lithium aluminum hydride and lithium borohydride
A technology of lithium aluminum hydride and lithium borohydride, applied in chemical instruments and methods, metal hydrides, inorganic chemistry, etc., can solve the problems of high cost, slow outgassing, decomposition, and inability to meet the requirements, and achieves low production cost and easy production. Operation, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 The method for preparing α-aluminum hydride with lithium aluminum hydride and lithium borohydride mixed catalysis comprises the following steps:
[0028] (1) Preparation of mixed catalyst solution: take LiAlH by weight 4 and LiBH 4 , placed in a reaction flask, added refined anhydrous diethyl ether, and fully stirred for 10 minutes to obtain a mixed catalyst solution for subsequent use;
[0029] (2) Synthesis of α-aluminum trihydride: Take 2000mL of refined toluene and place it in a 10L reaction vessel, and then pass nitrogen gas into the reaction vessel. After repeating twice, add 300mL of the mixture obtained in step (1) through vacuum filtration. Catalyst solution, under reflux state, stir and heat up, begin to distill out the diethyl ether in the catalyst, at this moment slowly add dropwise 350mLLiAlH 4 and anhydrous AlCl 3 Mix the solution, control the dropping rate at 10ml / min, after the dropwise addition is completed, raise the temperature to 85°C...
Embodiment 2
[0033] Embodiment 2 The method for preparing α-aluminum trihydride with lithium aluminum hydride and lithium borohydride mixed catalysis, comprises the following steps:
[0034] (1) Preparation of mixed catalyst solution: take LiAlH by weight 4 and LiBH 4 , placed in a reaction flask, added refined anhydrous diethyl ether, and fully stirred for 50 minutes to obtain a mixed catalyst solution for subsequent use;
[0035] (2) Synthesis of α-aluminum trihydride: take 3000mL of refined toluene and place it in a 10L reaction vessel, and pass nitrogen gas into the reaction vessel. After repeating 4 times, add 200mL of the mixture obtained in step (1) through vacuum filtration. Catalyst solution, under reflux state, stir and heat up, begin to distill out the diethyl ether in the catalyst, at this moment slowly add dropwise 500mLLiAlH 4 and anhydrous AlCl 3 Mix the solution, control the rate of addition to 20ml / min, after the dropwise addition is completed, heat up to 95°C and reflu...
Embodiment 3
[0040] Embodiment 3 The method for preparing α-aluminum trihydride with lithium aluminum hydride and lithium borohydride mixed catalysis, comprises the following steps:
[0041] (1) Preparation of mixed catalyst solution: take LiAlH by weight 4 and LiBH 4 , placed in a reaction flask, added refined anhydrous diethyl ether, and fully stirred for 30 minutes to obtain a mixed catalyst solution for subsequent use;
[0042] (2) Synthesis of α-aluminum trihydride: Take 2500mL of refined toluene and place it in a 10L reaction vessel, and then pass nitrogen gas into the reaction vessel. After repeating 3 times, add 250mL of the mixture obtained in step (1) through vacuum filtration. Catalyst solution, under reflux state, stir and heat up, begin to distill out the diethyl ether in the catalyst, at this moment slowly add dropwise 450mLLiAlH 4 and anhydrous AlCl 3 Mix the solution, control the dropping rate at 15ml / min, after the dropwise addition is completed, raise the temperature t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com