Preparation method of transition metal phosphide with good morphology
A transition metal and phosphide technology, applied in phosphide and other directions, can solve the problems of inability to prepare phosphide, poor catalytic activity of the product, mixed crystal phase of the product, etc., and achieve the effect of being suitable for large-scale production, low cost and good crystalline performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 6.0g of cobalt chloride hexahydrate and 6.6g of sodium hypophosphite hydrate, and mix them evenly in a mortar; react the mixture at 800°C under the protection of argon for 60min; grind the obtained product into fine powder, wash with distilled water and absolute ethanol three times respectively , and then dried under vacuum at 60°C for 12 hours to obtain the product CoP.
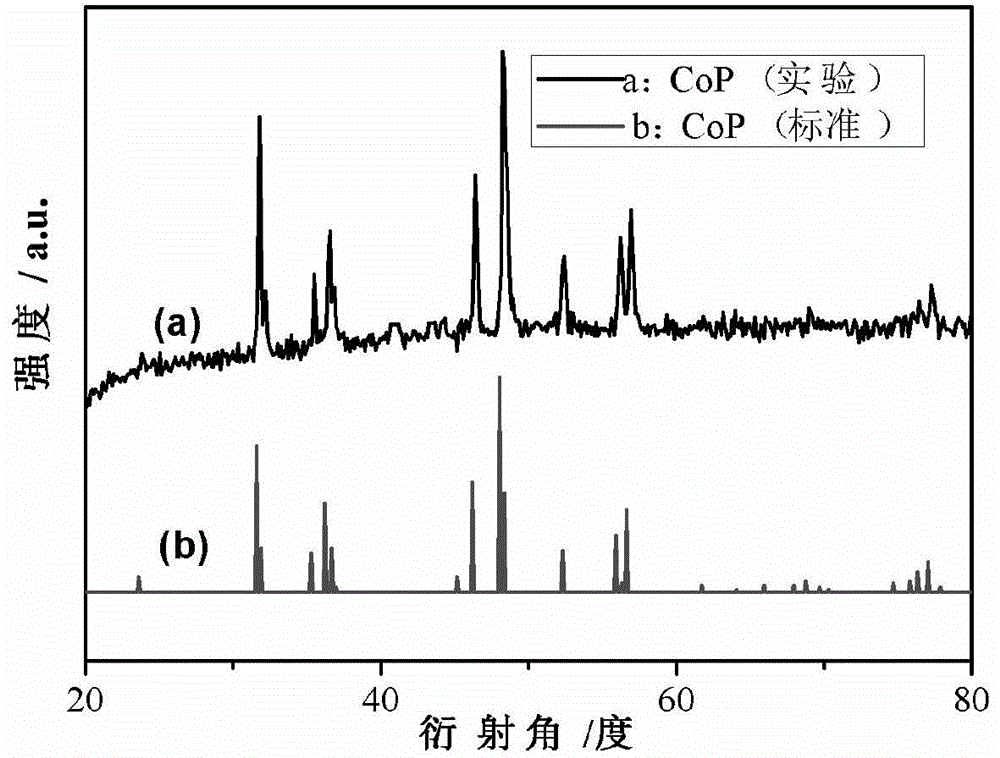
[0035] figure 1 This is the XRD pattern of the obtained material. It can be seen from the comparison with the standard card that the synthesized material is a standard CoP.
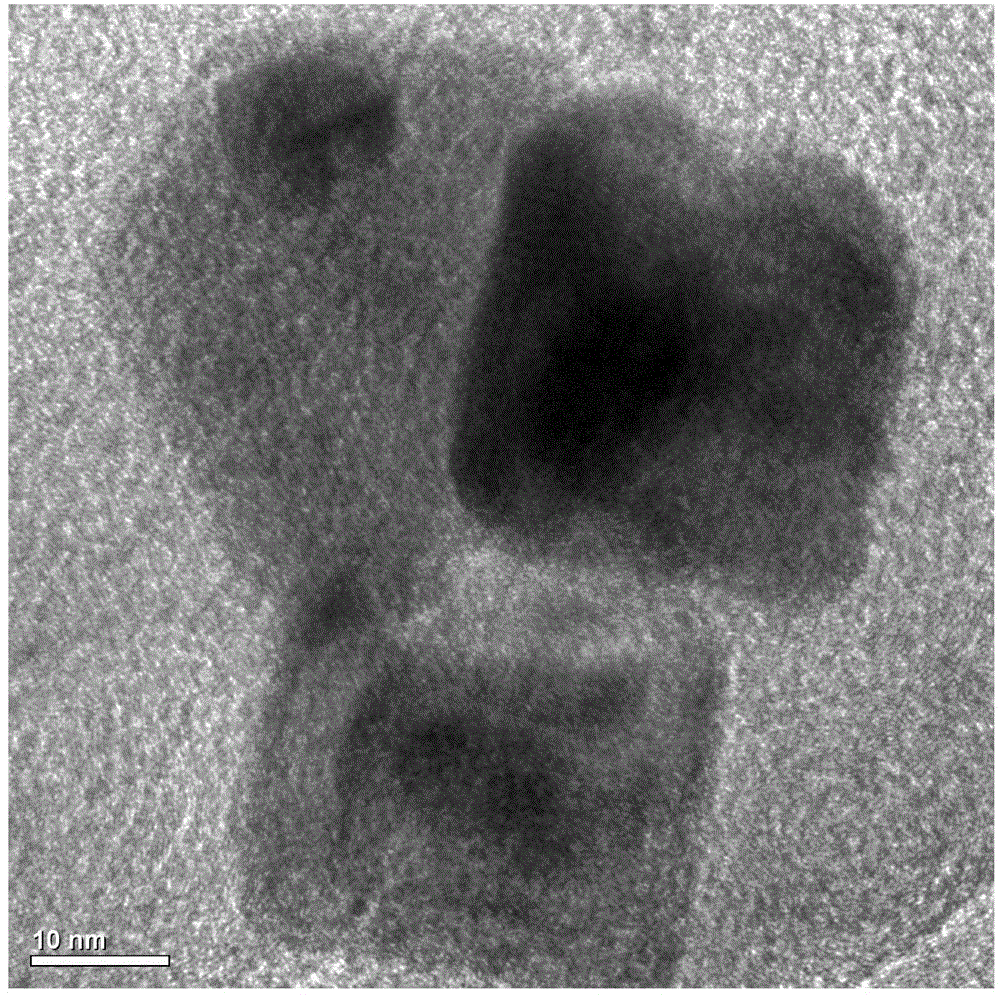
[0036] figure 2 It is the TEM image of the prepared CoP. It can be seen from the figure that the CoP has a square regular structure.
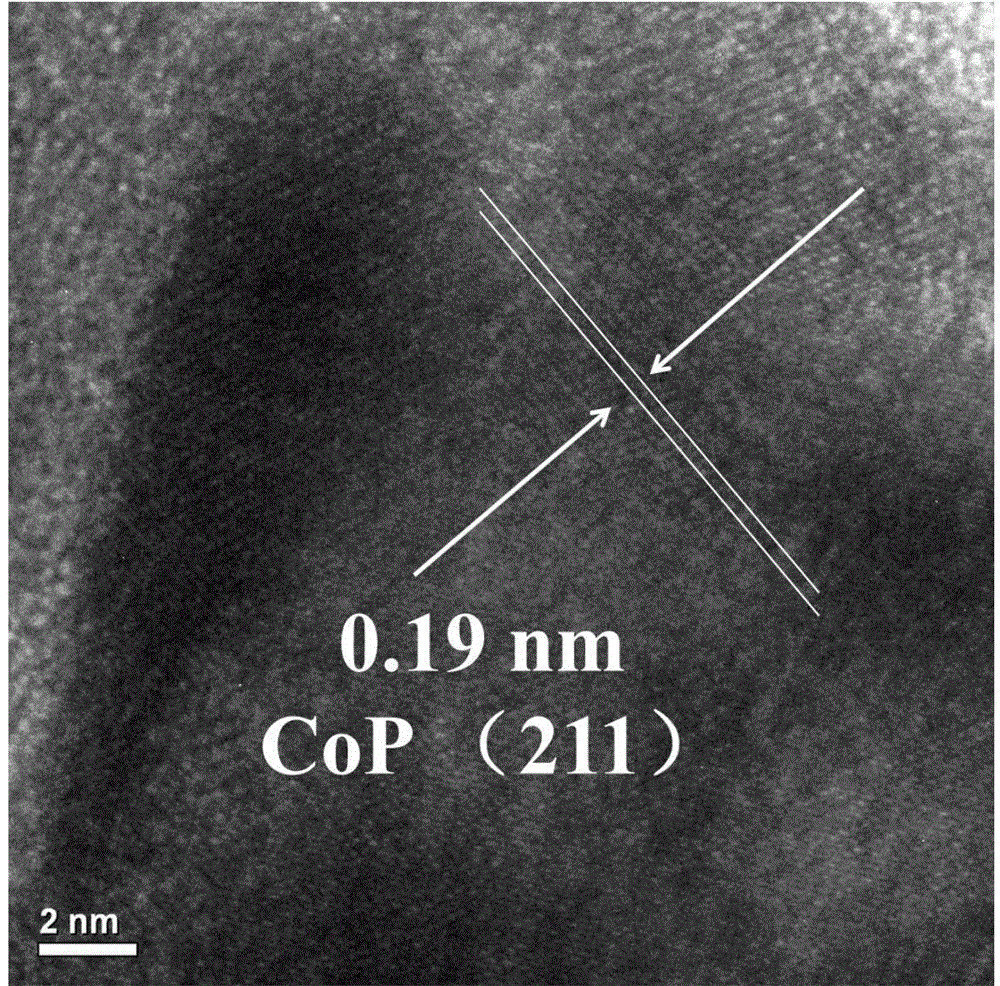
[0037] image 3 It is the HR-TEM image of the prepared CoP. It can be seen from the figure that the lattice fringes of CoP are very obvious, and CoP is mainly stacked in face-centered cubic, and the exposed crystal plane is mainly (211) crystal plane, showing a single The crystal form, ...
Embodiment 2
[0039] Weigh 2.811g of cobalt sulfate heptahydrate and 1.255g of sodium hydrogen phosphite pentahydrate, and mix them uniformly in a mortar; react the mixture at 800°C under the protection of argon for 120min; grind the obtained product into fine powder, wash with distilled water and absolute ethanol three times respectively , and then vacuum dried at 80°C for 18h to obtain the product CoP.
[0040] Figure 4 This is the XRD pattern of the obtained material. It can be seen from the comparison with the standard card that the synthesized material is a standard CoP.
[0041] Figure 5 It is the TEM image of the prepared CoP. It can be seen from the figure that the CoP has a square regular structure.
[0042] Image 6 It is the HR-TEM image of the prepared CoP. It can be seen from the figure that the lattice fringes of CoP are very obvious, and CoP is mainly stacked in face-centered cubic, and the exposed crystal plane is mainly (211) crystal plane, showing a single The crysta...
Embodiment 3
[0044] Weigh 3.0g of nickel chloride hexahydrate and 6.6g of sodium hypophosphite hydrate, and mix them evenly in a mortar; react the mixture at 250°C under the protection of argon for 60min; grind the obtained product into fine powder, wash with distilled water and absolute ethanol three times respectively , and then vacuum dried at 60°C for 12h to obtain the product Ni 2 p.
[0045] Figure 7 It is the XRD pattern of the obtained material. It can be seen from the standard card that the synthesized material is the standard Ni 2 p.
[0046] Figure 8 For the prepared Ni 2 The TEM image of P, as can be seen from the figure, Ni 2 P is a rectangular regular structure.
[0047] Figure 9 For the prepared Ni 2 The HR-TEM image of P, as can be seen from the figure, Ni 2 The lattice fringes of P are very obvious, and Ni 2 P mainly stacks in the form of face-centered cubic, and the exposed crystal planes are mainly (111) crystal planes, and the crystal planes present a singl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com