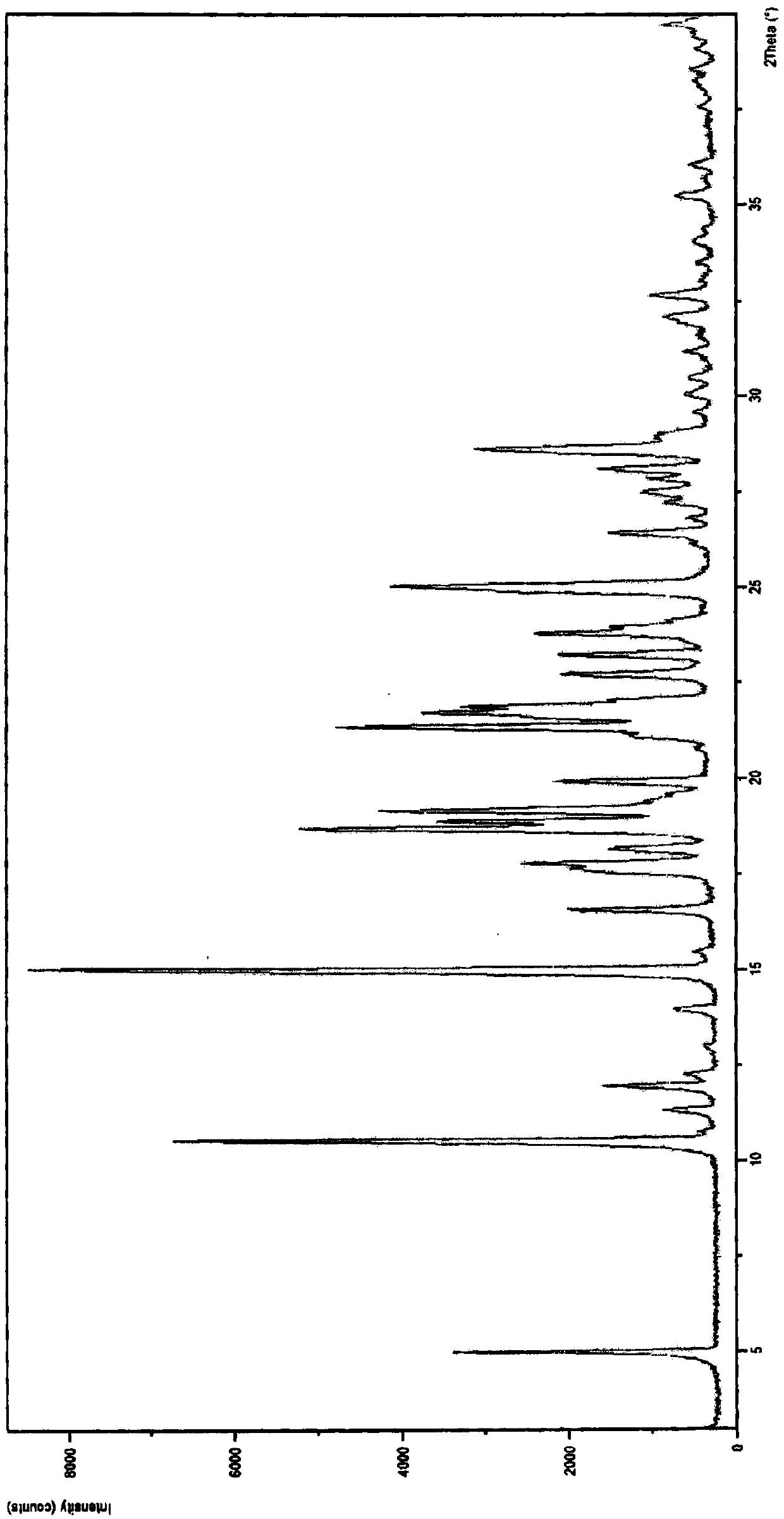
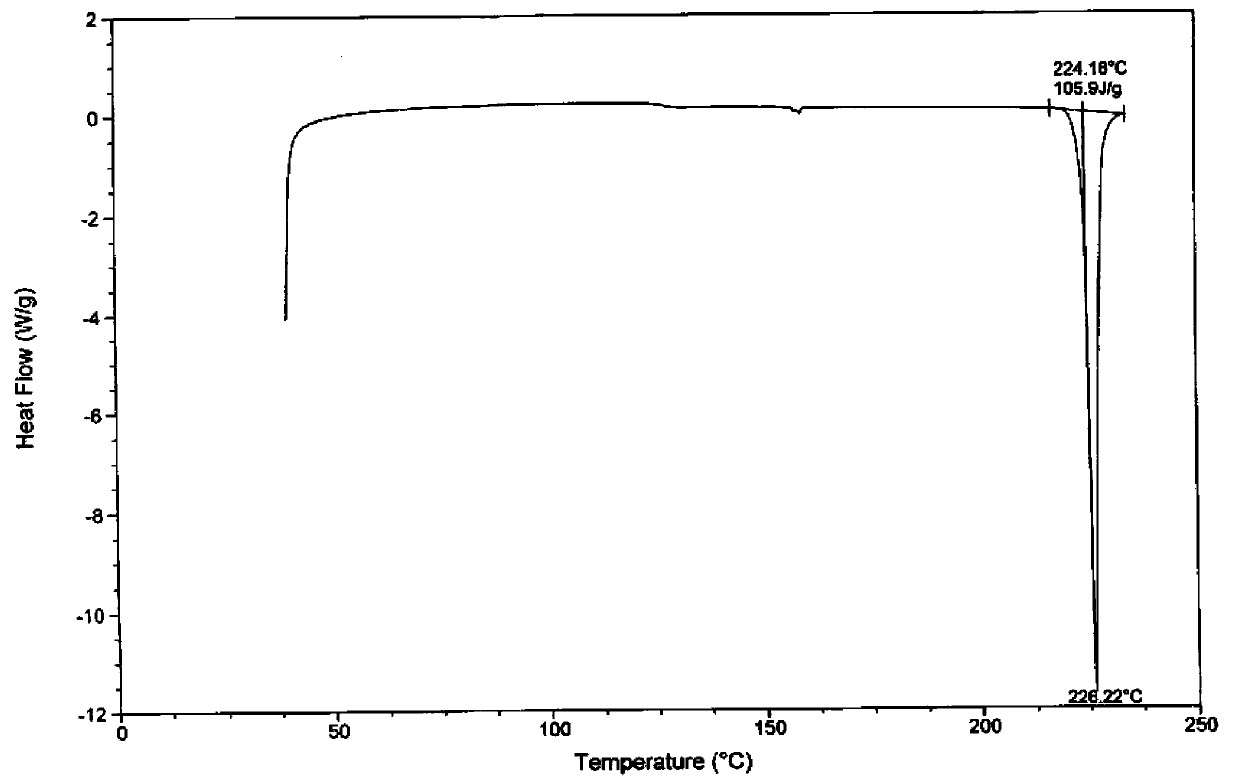
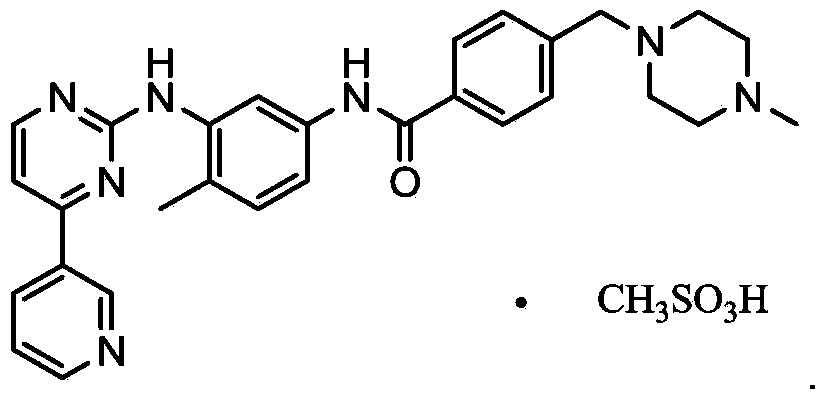
Preparation method of imatinib mesylate alpha crystal form
A technology of imatinib mesylate and imatinib base, which is applied in the field of preparation of polymorphic drugs, can solve the problems of unqualified solvent residues, and achieve the effects of solving solvent residues, single crystal form, and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of imatinib mesylate α crystal form
[0032] Take imatinib base 5.0g, add 12.0mL dimethyl sulfoxide, stir and heat to 85°C to completely dissolve, cool down to 40°C, add 0.9725g (1eq.) methanesulfonic acid, stir for 1h, heat up to 60°C, add 75mg of imatinib mesylate in the α crystal form was used as the initiation seed crystal, and 180ml of isopropanol was slowly added dropwise, cooled to 25°C, stirred and crystallized for 10h, filtered and dried to obtain 5.4g of off-white powder, with a purity of 99.70%, and a yield of 90% %. Embodiment 2: the preparation of imatinib mesylate α crystal form
Embodiment 2
[0032] Take imatinib base 5.0g, add 12.0mL dimethyl sulfoxide, stir and heat to 85°C to completely dissolve, cool down to 40°C, add 0.9725g (1eq.) methanesulfonic acid, stir for 1h, heat up to 60°C, add 75mg of imatinib mesylate in the α crystal form was used as the initiation seed crystal, and 180ml of isopropanol was slowly added dropwise, cooled to 25°C, stirred and crystallized for 10h, filtered and dried to obtain 5.4g of off-white powder, with a purity of 99.70%, and a yield of 90% %. Embodiment 2: the preparation of imatinib mesylate α crystal form
[0033] At room temperature, take 10.0g of imatinib base, add 90mL of ethanol, slowly dropwise add 1.94g of ethanol solution of methanesulfonic acid, after the addition is complete, continue to stir for 3-5h, filter and dry to obtain imatinib methanesulfonic acid Salt coarse product.
[0034] Take 5.0g of the crude product of imatinib mesylate, add 10.0mL of dimethyl sulfoxide, stir and heat to 85°C to dissolve completely,...
Embodiment 4
[0038] Embodiment 4: measure the residual solvent of product in embodiment 1-3
[0039] Determination of isopropanol dissolution residue:
[0040] Take about 0.1g of the test sample, accurately weigh it into a 20mL headspace bottle, add 1.0mL of dimethylformamide accurately, dissolve it by ultrasonic, and use it as the test solution. According to the residual solvent determination method (Chinese Pharmacopoeia 2010 edition two appendix VIIIP second method) test. Adopt DB-624 (30m×0.53mm×3.0μm) chromatographic column, temperature program, the initial temperature is 40°C, keep for 5 minutes, and rise to 220°C at a rate of 20°C per minute; the detector is FID, and the temperature is 250°C ; The temperature of the injection port is 160°C; the carrier gas is nitrogen; headspace injection, the headspace equilibrium temperature is 80°C, and the equilibrium time is 30min.
[0041] Determination of dimethylformamide and dimethyl sulfoxide dissolution residues:
[0042] Take about 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com