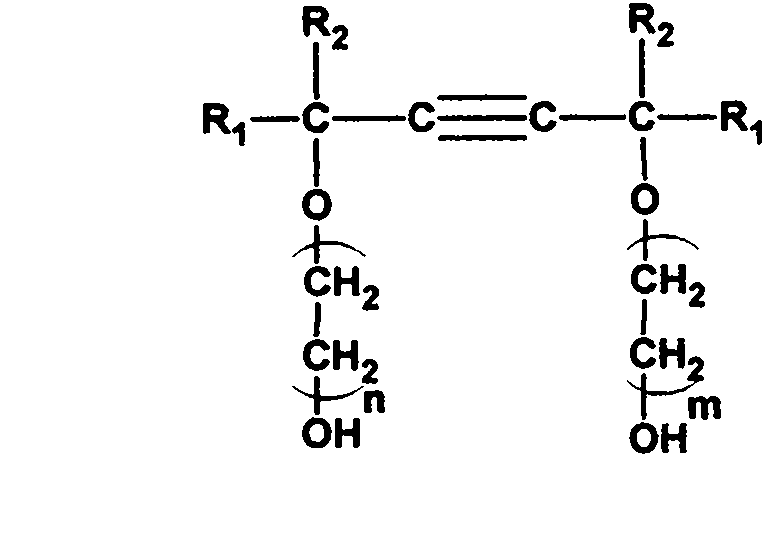
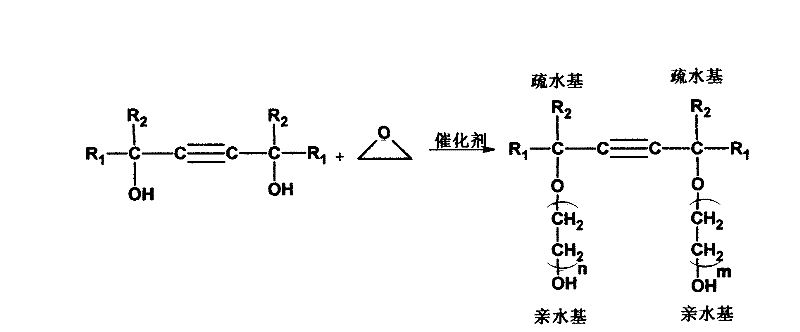
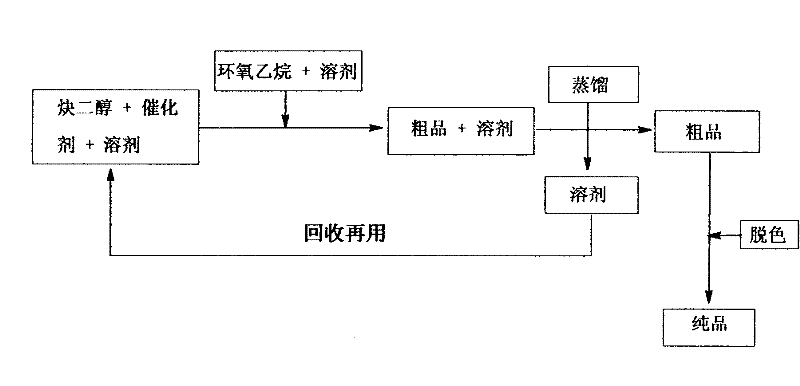
Method for synthesizing adduct of alkynediol and epoxyethane
A technology for the synthesis of ethylene oxide, which is applied in the preparation of ether from alkylene oxide, chemical instruments and methods, transportation and packaging, etc. It can solve the problems of complex operation, large equipment investment, and difficult control of reaction temperature, etc., and achieve production safety Improve performance, reduce equipment investment, and reduce volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct (1:1.5).
[0034] Add 226 grams (1.0mol) of 2,4,7,9-tetramethyl-5-decyne-4,7-diol in a 5-liter three-necked round-bottomed flask, 500 milliliters of anhydrous ether and 0.1mol Under reflux with mechanical stirring, slowly add dropwise a mixed solution of 1.5 mol of ethylene oxide dissolved in 500 ml of anhydrous diethyl ether, after the dropwise addition, continue to stir and reflux for 5 hours. After the reaction is completed, anhydrous ether is removed by rotary evaporation, and a small amount of trimethylamine is extracted under reduced pressure to obtain 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct compound (1:1.5), code-named CHEM-10.
Embodiment 2
[0036] Synthesis of 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct (1:3.5).
[0037] Add 316 g (1.0 mol) of CHEM-10, 500 ml of anhydrous ether and 0.1 mol of trimethylamine in a 5-liter three-necked round-bottomed flask, slowly add 2.0 mol of ethylene oxide dropwise under mechanical stirring and reflux Dissolve the mixed solution in 500 ml of anhydrous ether, after the dropwise addition, continue to stir and reflux for 10 hours. After the reaction is completed, anhydrous ether is removed by rotary evaporation, and a small amount of trimethylamine is extracted under reduced pressure to obtain 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct compound (1:3.5), code-named CHEM-20.
Embodiment 3
[0039]Synthesis of 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct (1:10).
[0040] Add 436 grams (1.0mol) of CHEM-20, 500 milliliters of anhydrous ether and 0.1mol of trimethylamine in a 5-liter three-necked round-bottomed flask, slowly add 6.5mol of ethylene oxide dropwise under mechanical stirring and reflux Dissolve the mixed solution in 1000 ml of anhydrous ether, after the dropwise addition, continue to stir and reflux for 20 hours. After the reaction is completed, anhydrous ether is removed by rotary evaporation, and a small amount of trimethylamine is extracted under reduced pressure to obtain 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethylene oxide adduct compound (1:10), code-named CHEM-30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com