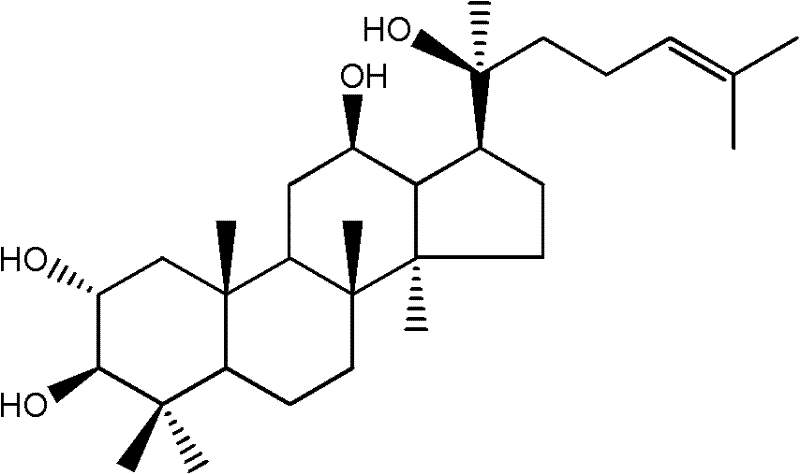
Application of 2 alpha-hydroxy protopanoxadiol medicine
A panaxadiol and drug technology, which is applied to the application field of 2α-hydroxy protopanaxadiol in the preparation of antitumor drugs, can solve few problems and the like, and achieve the effects of inhibiting proliferation and inhibiting tumor significantly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Determination of the inhibitory effect of 2α-hydroxyprotopanaxadiol on the growth of pancreatic cancer cells by MTS method
[0035] 3000 PANC-1 cells (purchased from the Chinese Academy of Sciences Cell Bank) were inoculated into 96-well plates, cultured for 24 hours to allow them to adhere to the wall, and then 2α-hydroxyprotopanaxadiol (purchased from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences) was added. 6 concentration gradients, with 3 replicate wells for each concentration. Cells at 37°C, 5% CO 2 After culturing for 72 hours under the same conditions, the culture medium was discarded, and the cell viability was measured with an MTS kit (Promega).
[0036] The test method is: wash the cells once with serum-free medium, and add the pre-prepared MTS chromogenic solution according to the amount of 100 μl / well (add 2ml of solution 1 and 100μl of solution 2 to 10ml of serum-free medium, and mix well). A well without cells was set a...
Embodiment 2
[0039] Example 2 Growth inhibitory effect of 2α-hydroxyprotopanaxadiol on human leukemia cells
[0040] Using cck-8 kit (Japan Dojin Chemical Research Institute) detection.
[0041] steps:
[0042] 1) K562 cells (purchased from ATCC) were evenly seeded in a 96-well plate, with 10,000 cells per well.
[0043] 2) Leave to adhere to the wall, add drugs after overnight, add drugs (2α-hydroxyprotopanaxadiol concentrations are 50, 16.67, 5.56, 1.85, 0.62 μM, and each concentration has 3 replicate wells.
[0044] 3) After culturing for 48 hours, replace the complete medium with a mixture of serum-free medium and CCK8 (10:1), and incubate in a 37°C incubator for 2 hours.
[0045] 4) Take 450nm as the measurement wavelength and 650nm as the reference wavelength, and measure the reading on a microplate reader.
[0046] Results: The IC50 value of 2α-hydroxyprotopanaxadiol on k562 cells was 4.77μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com