Analysis and assay of glycated hemoglobins by capillary electrophoresis, buffer compositions, and kits for capillary electrophoresis
A technology of glycosylated hemoglobin and capillary electrophoresis, which is applied in the field of analysis and determination of glycosylated hemoglobin to achieve the effect of reliable reading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
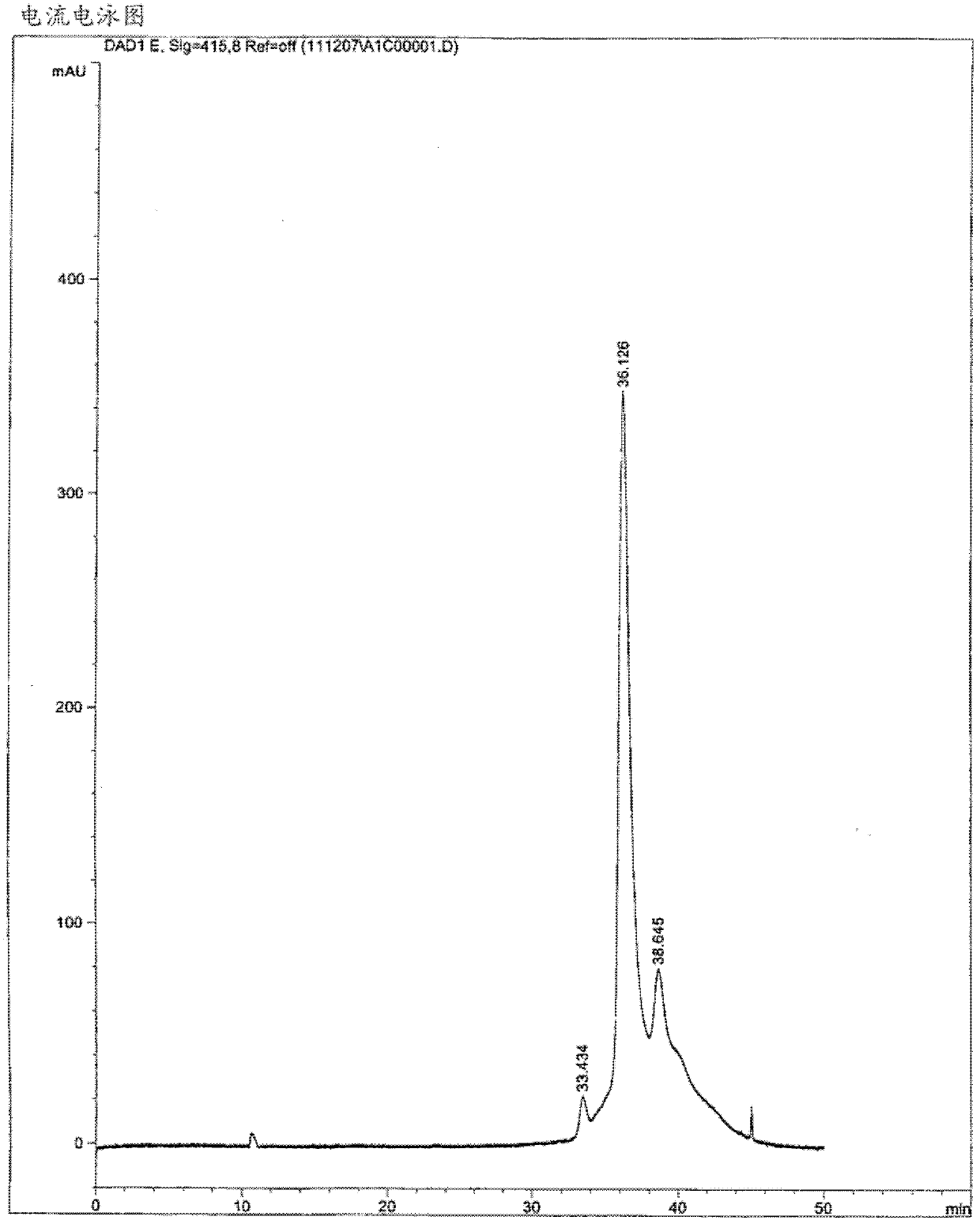
[0228] Normal human blood (containing hemoglobin HbA) diluted to 1 / 6 in hemolysis solution (1 g / L TritonX100 dissolved in 0 , HbA 1 and HbA 2 ) Capillary electrophoresis was performed using the assay buffer described in patent US5,599,433, which contains 100 mM CAPS and 300 mM orthoboric acid at a pH of 11. The obtained electropherograms are shown in figure 1 middle. The separation between the peaks for hemoglobin is less pronounced.
Embodiment 2
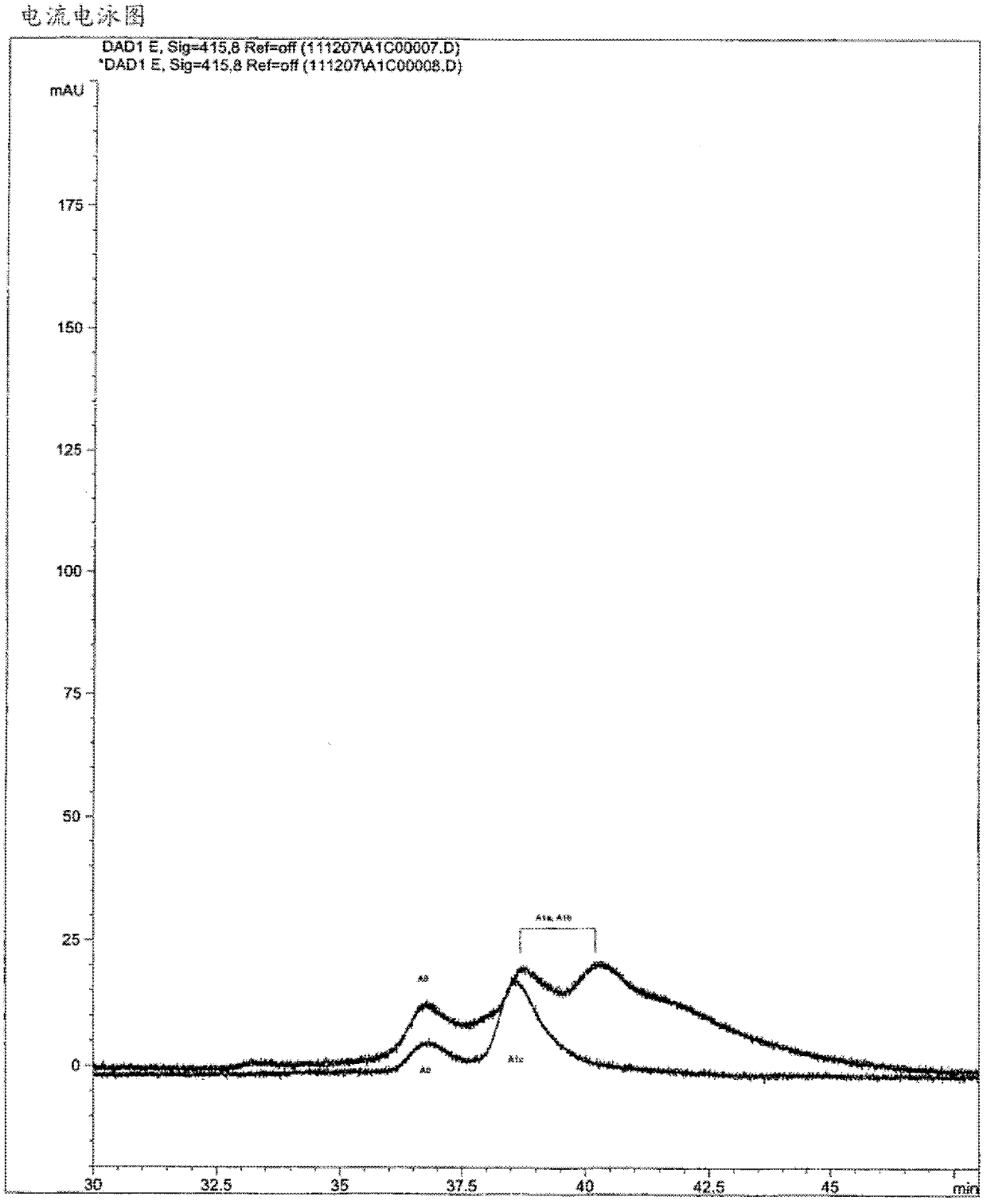
[0230] From different purified fractions containing glycosylated hemoglobin (fraction A 0 and A 1c or Part A 0 、A 1b and A 1a ) reference sample (Exocell, USA) was subjected to capillary electrophoresis using the analytical buffer described in the patent US5,599,433, which contained 100 mM CAPS and 300 mM orthoboric acid, pH 10.20. Income A 1a 、A 1b and A 1c The standard electrophoretic curve is shown in figure 2 middle. HbA 1c with HbA 1a 、A 1b Significantly insufficient separation between hemoglobins; HbA 1c Electrophoretic peak covering HbA1 a / HbA1 b peak.
Embodiment 3
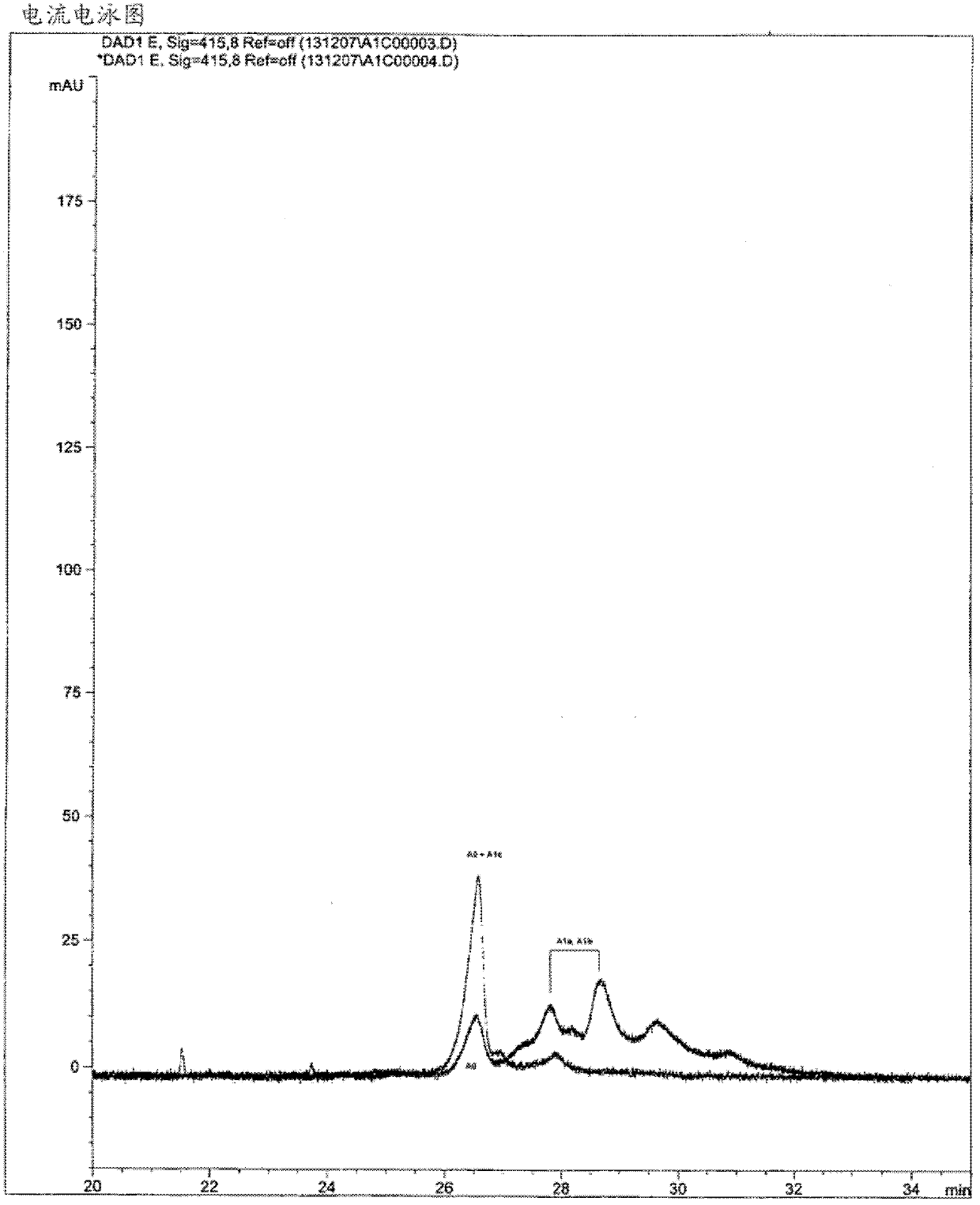
[0232] From different purified fractions containing glycosylated hemoglobin (fraction A 0 and A 1c or Part A 0 、A 1b and A 1a ) reference sample (Exocell, USA) was subjected to capillary electrophoresis using a buffer composition containing 200 mM CAPSO and 10 mM putrescine (pH 10.20), but neither an orthoborate compound nor a borate-based compound . Income A 1a 、A 1b and A 1c The standard electrophoretic curve is shown in image 3 middle. Corresponds to HbA1 c peak of HbA o peaks co-migrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com