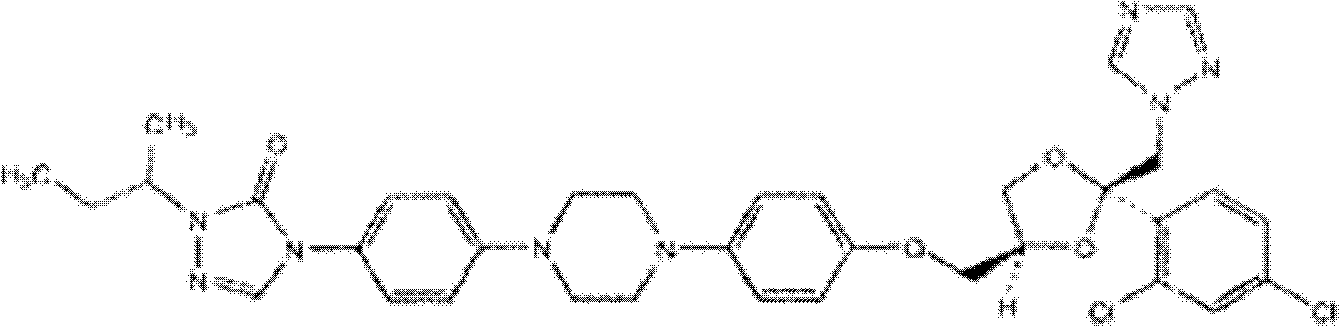
Itraconazole dispersible tablets and preparation method thereof
A technology of itraconazole and dispersible tablets, applied in the field of itraconazole dispersible tablets and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Accurately weigh the following ingredients:
[0024] Itraconazole 100g (Shandong Shouguang Fukang Pharmaceutical Co., Ltd.), hydroxypropyl methylcellulose 100g (Anhui Shanhe Pharmaceutical Excipients Company), crospovidone (purchased from BASF) 100g, dichloromethane 1000g (Tianjin Fuchen Chemical Reagent Factory). Mix the raw materials evenly, control the solution temperature to 40°C, and let itraconazole and other solids be dissolved in liquid dichloromethane.
[0025] Accurately weigh the following ingredients:
[0026] Cyclodextrin 100g, sodium lauryl sulfate 10g, polyethylene glycol 400010g, absolute ethanol 300g. Mix the raw materials evenly, control the solution temperature to 40°C, and let the solids such as cyclodextrin dissolve in the liquid ethanol.
[0027] Mix the above two solutions evenly until itraconazole is completely dissolved, and then dry it with a spray dryer, the temperature at the inlet of the dryer is 170°C, and the temperature at the outlet i...
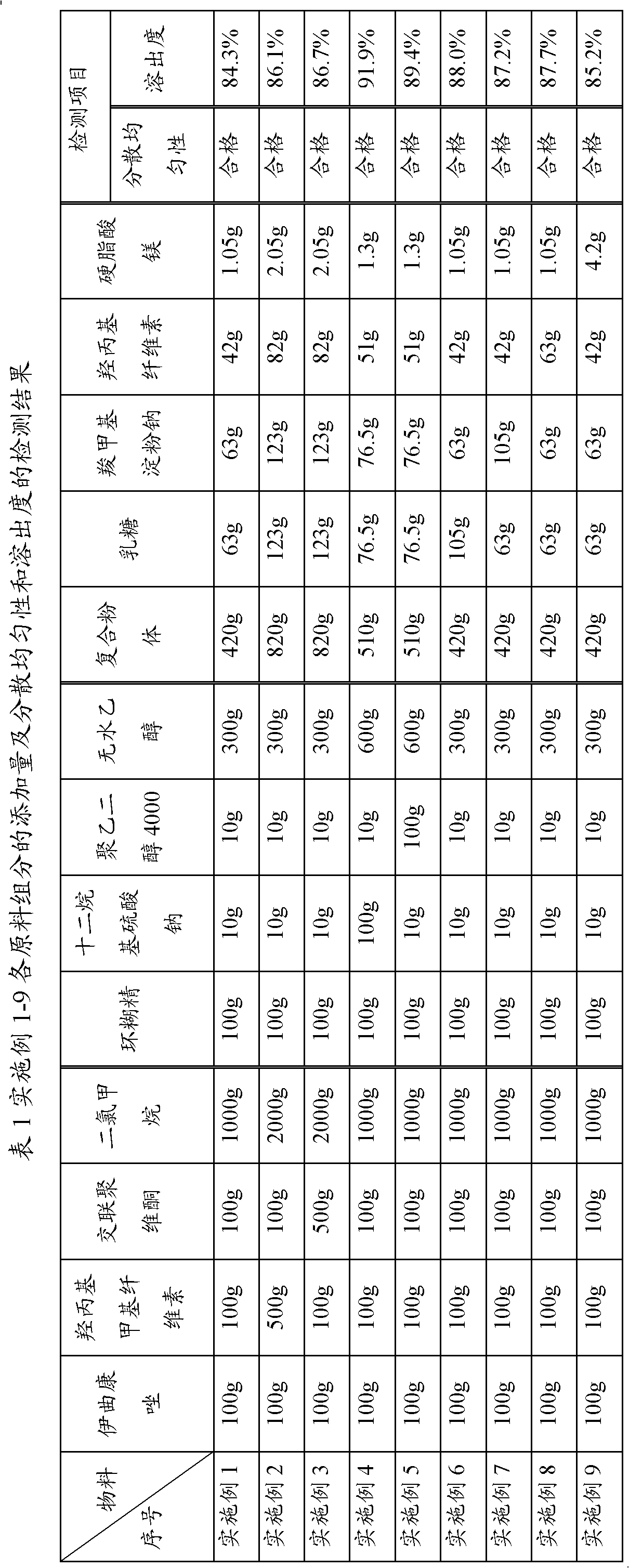
Embodiment 2 to 9
[0030] Examples 2 to 9, as shown in Table 1: the operation steps are the same as in Example 1, except that the addition amount of each component is different. In addition, for the process conditions in the preparation method of the present invention (such as the dissolution temperature of each step described in the foregoing summary of the invention, the temperature of the inlet and outlet of the drier, the temperature of the inlet and outlet of the granulator, the selection type of the granulator, etc.), it can be based on the addition of Simple adjustments to the amounts of components or actual operating conditions have basically no effect on the properties of the obtained itraconazole dispersible tablets.
[0031] The selection and weight ratio of each component in the preparation method of itraconazole dispersible tablet of the present invention is the preferred range value obtained by the inventor through consulting a large number of documents and numerous tests, that is, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com