SCX/HIC (Strong Cation Exchange/Hydrophobic) mixed-mode chromatograph stationary phase and preparation method thereof
A mixed-mode chromatography and stationary phase technology is applied to a hydrophobic/strong cation-exchange dual-functional mixed-mode chromatography stationary phase. In the field of preparation of the mixed-mode stationary phase, problems such as limited types can be solved, and the synthesis cost is low and the service life is long. , good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
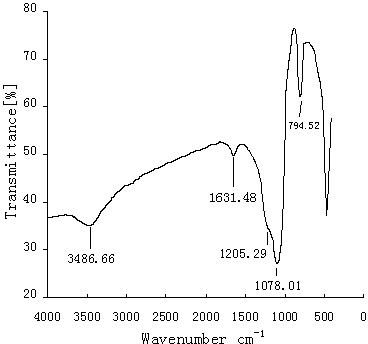
[0046] (1) Take 3.3g (about 13.7 mmol) of cystine and dissolve it in 150 mL of 0.5 mol / L Na 2 CO 3 buffer solution, then adjust the pH to 11.0, add it to a 250 mL three-necked flask, and slowly add 3 mL (about 13.7 mmol) of TM560 dropwise under ice-bath stirring. 65°C, reacted for 24 hours, the reaction was completed, the solution was light yellow, cooled to room temperature, adjusted the pH of the reaction solution to 5.5 with glacial acetic acid, added 2 g of silica gel, reacted at 90°C for 2 hours, filtered, and washed three times with water, methanol, Wash with acetone and methanol, and dry the obtained solid under vacuum at 50° C. for 8 hours to obtain a cystine-bonded silica gel derivative. Take 5.0 mL of ninhydrin ethanol solution, add a small amount of cystine silica gel derivative, heat at 90°C for 5 min, and find that the solution turns purple, indicating that cystine has been successfully bonded to the silica gel surface.
[0047] (2) Disperse 2.0 g of cystine-bo...
Embodiment 2
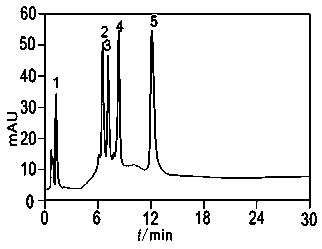
[0055] The chromatographic filler prepared in Example 1 was used to pack the column, and then the five standard proteins were separated under the strong cation exchange mode. Separation conditions:
[0056] Mobile phase: Liquid A: 20 mmol / L KH 2 PO 4 (pH 6.5); B liquid: 20 mmol / L KH 2 PO 4 + 1.0 mol / L NaCl (pH 6.5), linear gradient elution, 0→100%B, 30 min; flow rate 1.0 mL / min, for five proteins including myoglobin, RNase B, RNase A, cytochrome c, and lysozyme good separation (as figure 2 1, 2, 3, 4, and 5 shown are myoglobin, RNase B, RNase A, cytochrome c, and lysozyme, respectively).
Embodiment 3
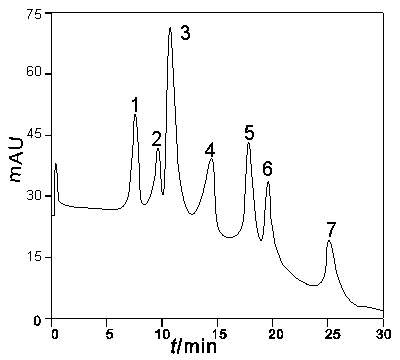
[0058] The chromatographic filler prepared in Example 1 was used to pack the column, and then the seven standard proteins were separated in the hydrophobic mode. Separation conditions:
[0059] Mobile phase: Liquid A: 20 mmol / L KH 2 PO 4 (pH 7.0) + 3.0 mol / L (NH 4 ) 2 SO 4 (pH 7.0); Solution B: 20 mmol / L KH 2 PO 4 (pH 7.0), linear gradient elution, 0→100%B, 30 min, flow rate of 1.5 mL / min, achieved cytchrome c, myoglobin, RNase A, lysozyme, OVA, α-amylase, insulin and other seven proteins good separation (eg image 3 1, 2, 3, 4, 5, 6, and 7 shown are cytchrome c, myoglobin, RNase A, lysozyme, OVA, α-amylase, insulin, respectively).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com