Transdermal pharmaceutical preparations
A technology for pharmaceutical preparations and pharmaceutical activity, applied in the field of transdermal pharmaceutical preparations, can solve problems such as microbial contamination, reduction of preparation stability and storage period, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
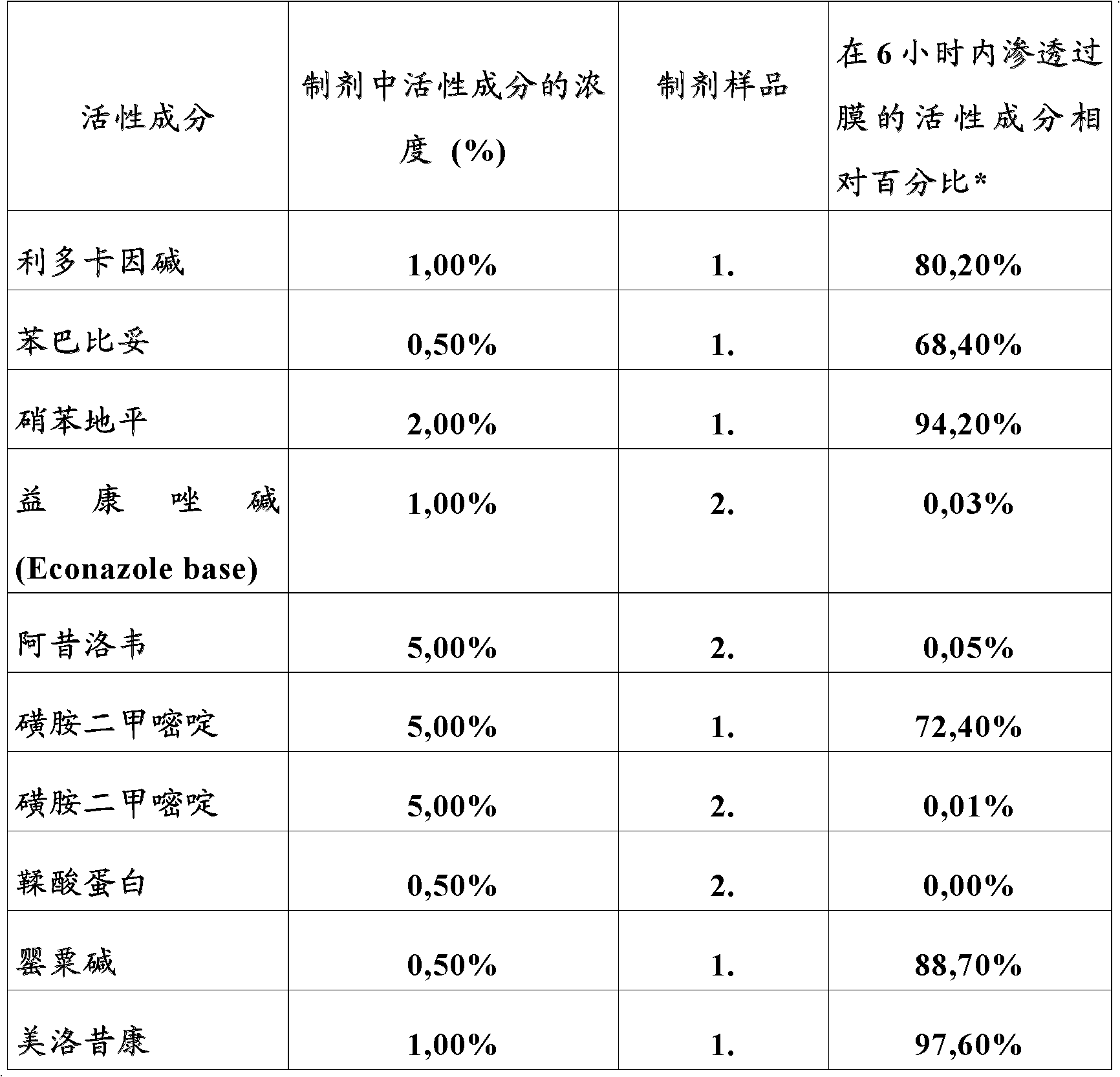
Image
Examples
Embodiment 1
[0074] Transdermal gel for systemic effects
[0075]
[0076] The amount of active ingredient is selected according to the desired formulation strength or according to the volume of reconstitution and the unit dose.
Embodiment 2
[0078] Transdermal semisolid formulations for topical application
[0079]
[0080] The amount of active ingredient is selected according to the desired formulation strength or according to the volume of reconstitution and the unit dose.
Embodiment 3
[0082] Preparation
[0083] Compositions according to example 1 or 2 and compositions of similar qualitative composition were prepared as follows.
[0084] 3.1. Preparation of active ingredient suspension
[0085] The optionally micronized active ingredient is mixed with silicone oil. The mixture is then homogenized with a suitable laboratory mixer, such as a laboratory-scale mixer, using an Ultra-Turrax mixing device (4000 min -1 , 5min).
[0086] 3.2. Preparation of gel matrix
[0087]Add hydroxypropyl cellulose to water in small portions at a temperature of 25°C and stir until completely dissolved. Carbopol 980NF was then added to the solution and stirred until dissolved. The solution was then neutralized with a 10% by weight potassium hydroxide solution. Stir continuously until a smooth gel state is obtained.
[0088] 3.3. Preparation of drug-containing gel
[0089] Add the active ingredient suspension in small portions to the gel base prepared according to 3.2 and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com