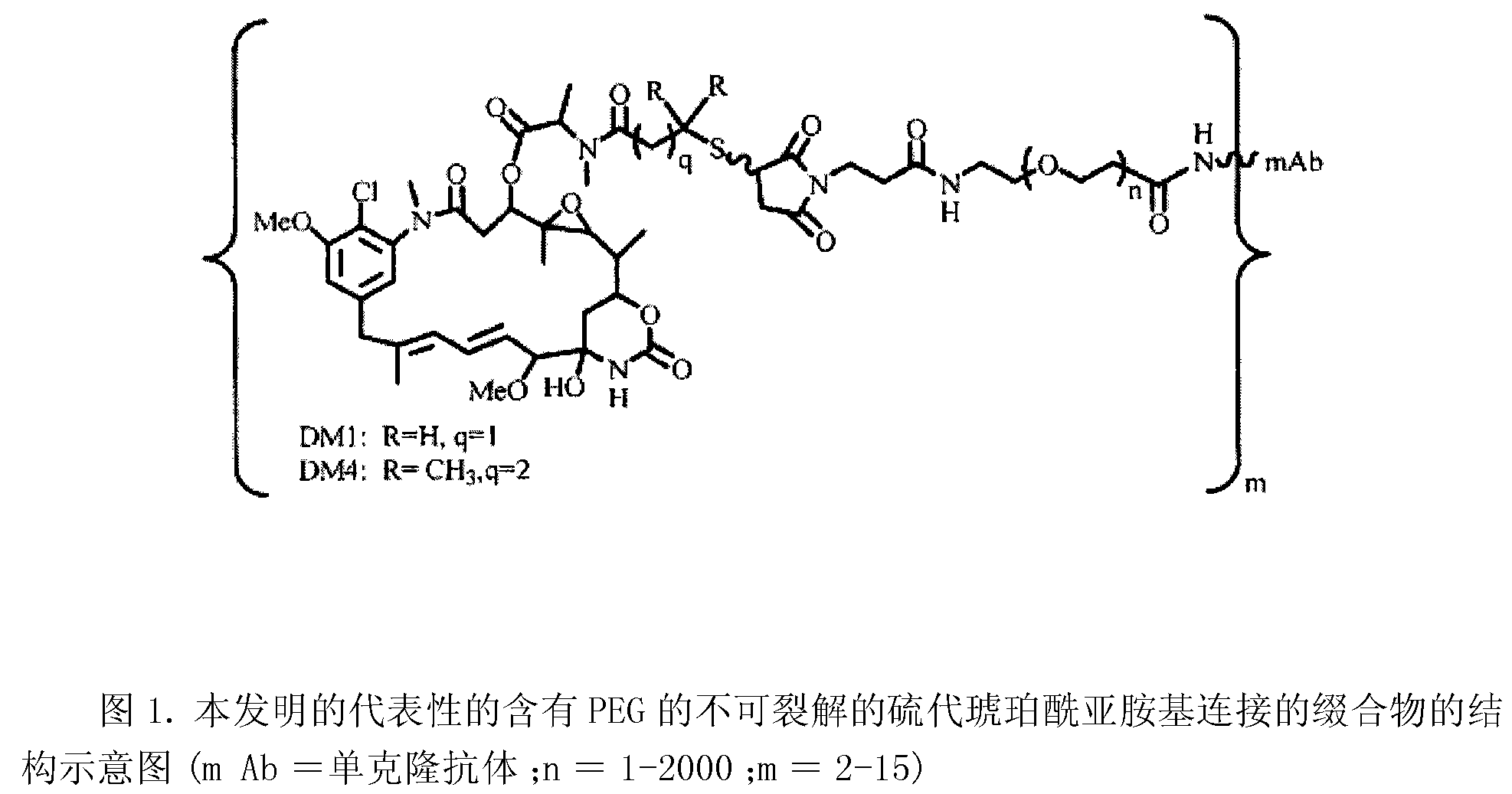
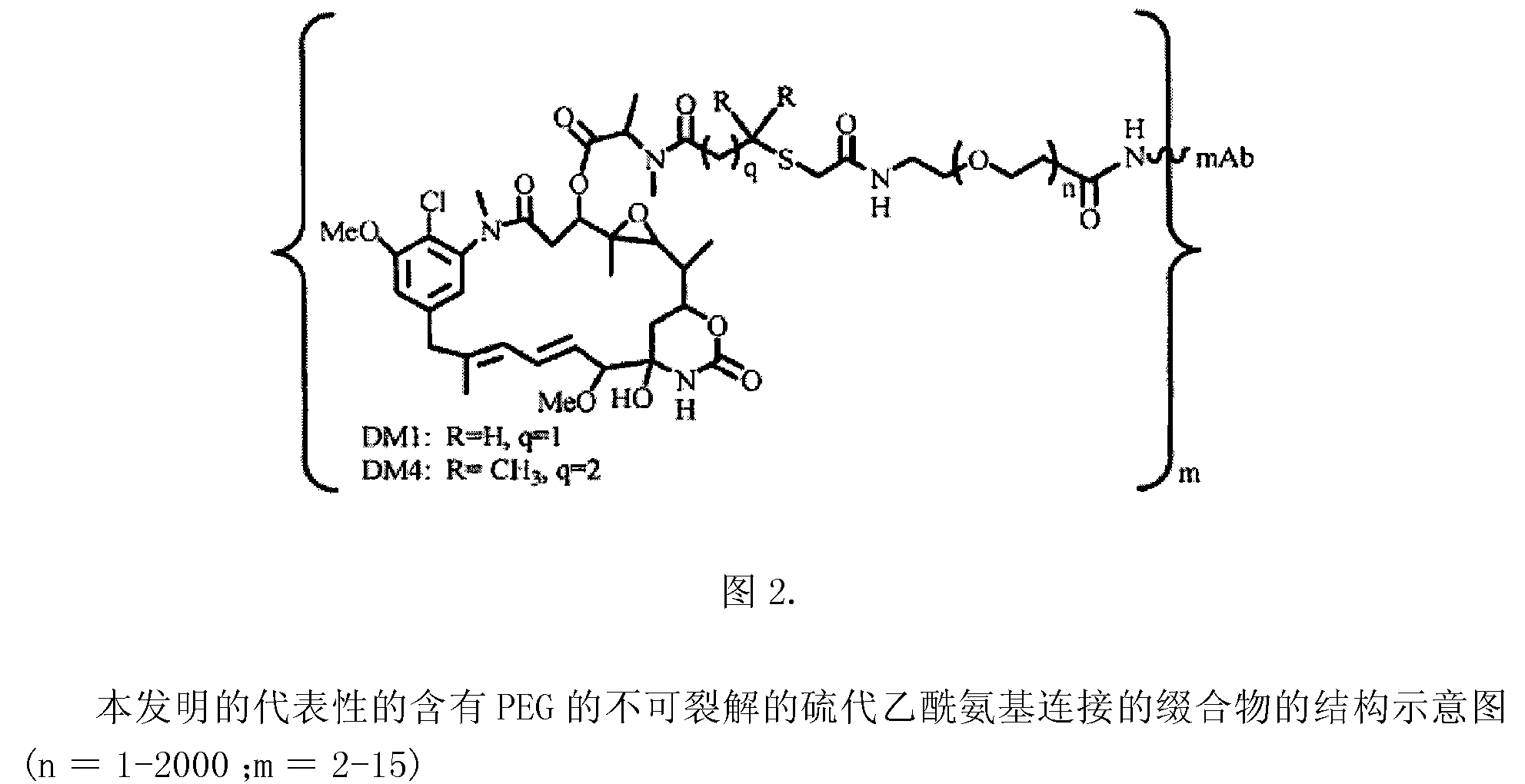
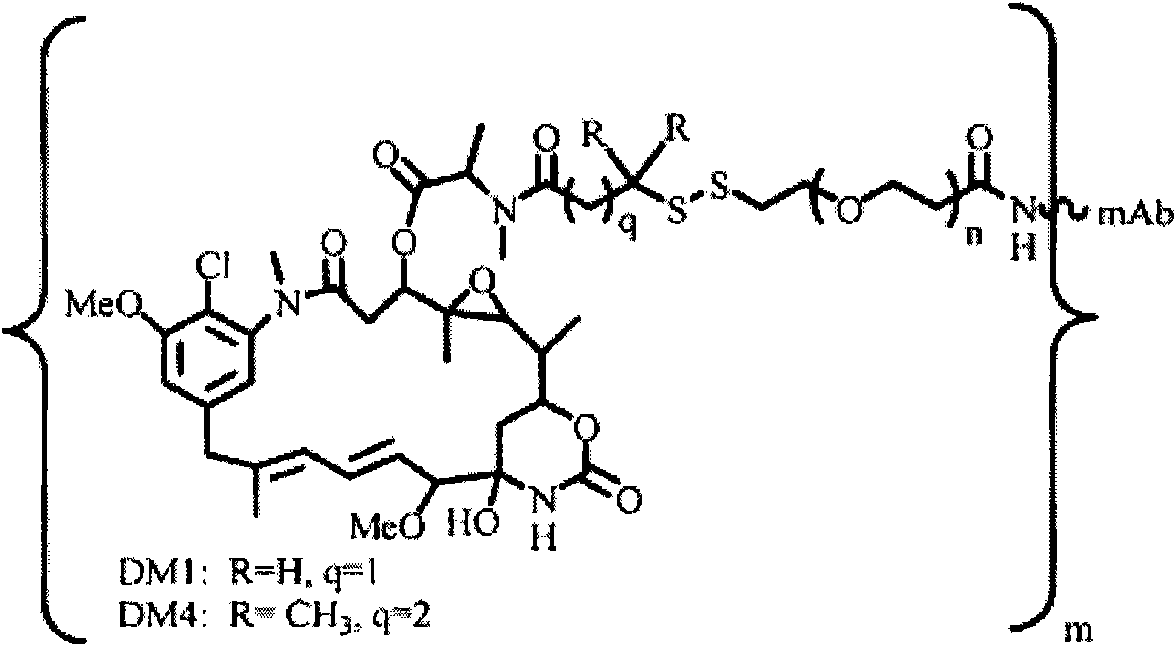
Potent conjugates and hydrophilic linkers
A technology of conjugates and compounds, applied in the field of hydrophilic linkers and preparation of maytansinoids, which can solve the problems of low yield of final conjugates, heterogeneity of conjugates, expensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0371] Without being bound by any particular aspect, polyethylene glycol ((CH) with different reactive linkers for conjugation with cell binding agents is described. 2 CH 2 O) n ) Synthetic method of the drug linked. These conjugation methods include via polyethylene glycol ((CH 2 CH 2 O) n ) one-step conjugation of a linker-linked antibody to a drug such as maytansinoid.
[0372] Furthermore, the synthesis of disulfide-containing polyethylene glycol ((CH 2 CH 2 θ) n ) method of linking the drug. These conjugation methods include the use of polyethylene glycol ((CH 2 CH 2 O) n ) one-step conjugation of the disulfide-linked antibody of the linker to a drug such as maytansinoid.
[0373] The following examples are illustrative only and are not intended to limit the invention.
Embodiment I
[0375] Antibodies with each anti- The body molecule is conjugated to several maytansinoid molecules:
[0376] In a two-step method of conjugating antibodies to several maytansinoid DM4 or DM1 molecules, a commercially available amine-reactive N-hydroxysuccinimide group (NHS group) and A heterobifunctional linker (SPDB) of a reactive 2-pyridyldithio group (-SSPy group) modifies the humanized antibody to incorporate several linker molecules into the antibody molecule (e.g. W.C. Widdison et al., J Med. Chem., 2006, 49, 4392-4408). After incorporation of the reactive linker into the antibody molecule, in a second reaction step, the maytansinoid DM4 or DM1, which has a reactive thiol group, is added to the linker-modified antibody to disulfide bond the maytansinoid Alkaloids are conjugated to antibodies. In a particular example, a 10-15 fold molar excess of commercially available methionine with -(CH 2 )- nAlkyl heterobifunctional linkers (such as SPDB, SPP, SPDP) modify th...
Embodiment II
[0378] By containing a hydrophilic polyethylene oxide spacer (PEG n or (-CH 2 -CH 2 -O) n=1-14 ) The disulfide linker makes the antibody linked to several maytansinoid molecules per antibody molecule Then conjugate:
[0379] In order to study hydrophilic spacers such as polyethylene oxide (PEG n or (-CH 2 -CH 2 -O) n=1-14 )) Whether it is possible to prevent the aggregation and precipitation of antibody maytansinoid conjugates with a large number of maytansinoid molecules (on average >4 per antibody molecule), several new heterobifunctional and monofunctional maytansinoid derivatives, which can be conjugated to antibodies by direct modification or a two-step reaction involving initial derivatization of antibodies at lysine residues followed by maytansinoid Alkaloid reactions (see, e.g. image 3 , 6, 11 and 12).
[0380] Synthesis of 15-(2-pyridyldithio)-4,7,10,13-tetraoxapentadecanoic acid
[0381] A solution of 2,2'-dipyridyldisulfide (aldri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com