Treatment using reprogrammed mature adult cells
A technology of reprogramming and cells, which is applied in the direction of extracellular fluid diseases, medical preparations containing active ingredients, and medical raw materials derived from mammals, etc. It can solve the problems of limited number of stem cells, troubles, and difficulties in stem cell differentiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
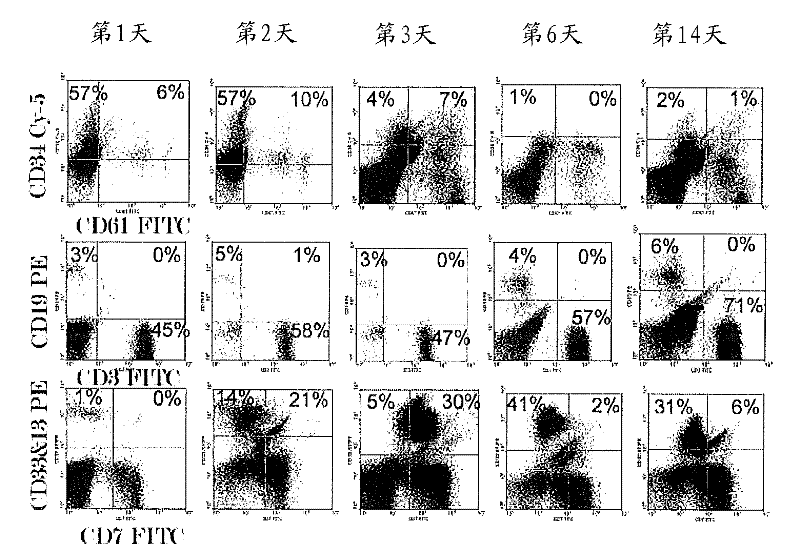
[0179] Materials and methods
[0180] This clinical study evaluated the safety of a single dose of autologous reprogrammed cells infused 3 hours after exposure to hematopoietic-inducing culture conditions into four aplastic anemia patients.
[0181] This clinical study was approved by the Ethics Committee of the King Edward Memorial (KEM) Hospital and conducted in collaboration with the Institute of Immunohematology (IIH). Patients were required to meet the criteria outlined in Table 3. As a result, four patients with severe (3 males) and aplastic (1 female) anemia were enrolled into the study. These 4 patients were selected and monitored by IIH / KEM staff. The patient's clinical and treatment history is described in Table 4, while his CD34 + Cell infusion doses are shown in Table 5.
[0182] Table 3: Inclusion Criteria
[0183]
[0184] Patients are transfused with 2 units of irradiated packed red blood cells and 4 units of platelets to maintain their hemoglobin levels...
Embodiment 2
[0221] Materials and methods
[0222] Autologous reprogrammed hematopoietic cells (target cells) were tested in 21 beta thalassemia patients. Nineteen patients had beta thalassemia major, while 2 patients had thalassemia intermedia. One of the β-thalassemia intermedia patients had a thalassemia / Hb E variant (commonly seen in patients of Far Eastern and Indian origin), while the other had thalassemia / sickle cell anemia.
[0223] Apheresis is performed on the patient by processing 2-3 times the total volume of the patient's blood. Autologous reprogrammed cells are produced by reprogramming leukocytes until target cells are obtained as indicated by their distinguishing features as described above. The autologous reprogrammed cells are administered to the patient by intravenous infusion into the jugular vein or a vein in the arm or thigh.
[0224] result
[0225]Following infusion of reprogrammed cells, no toxicity or adverse side effects were observed in beta thalassemia ...
Embodiment 3
[0229] Materials and methods
[0230] Apheresis was performed on two diabetic patients by processing 2-3 times the total amount of patient blood. Autologous reprogrammed mesenchymal stem cells, pluripotent stem cells and islet cells (target cells) were obtained by reprogramming apheresis leukocytes until the target cells developed as indicated by their distinguishing features as described above. The autologous reprogrammed cells are administered to the patient by intravenous infusion into the jugular vein or a vein in the arm or thigh.
[0231] result
[0232] Following infusion of autologous reprogrammed cells, patients synthesized normal levels of insulin, as measured by c-peptide stimulated by fasting and 90-minute food intake. This normal level of c-peptide was maintained until 3 months after infusion of the reprogrammed cells ( Figure 9 ). Furthermore, Hb A1C levels (which are indicative of glycemic control) were normalized after infusion of reprogrammed cells ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com