Method for improving anti-caking property of tebuconazole
A technology of tebuconazole and anti-caking, applied in the direction of organic chemistry, etc., can solve the problems of poor anti-caking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
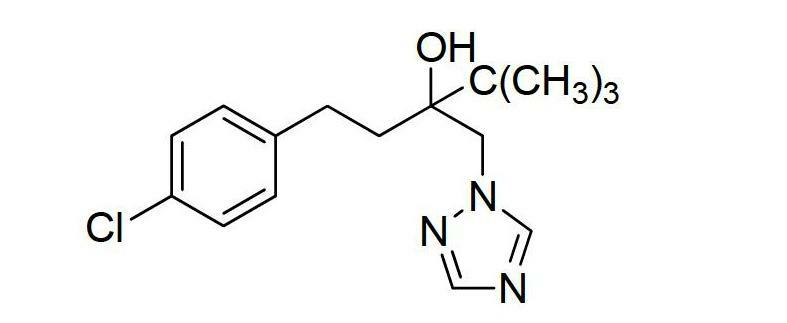
[0026] Preparation Example 1: 120 grams of 1-(4-chlorophenethyl)-1-tert-butyl-1,2-oxirane, 3 grams of potassium hydroxide, 40 grams of 1,2,4,-triazole, Add 1 g of N,N-dimethyl-4-aminopyridine and 100 ml of n-butanol into a 500 ml three-neck flask with a stirring and reflux tube, and stir for 6 hours at a reflux temperature of 120°C. After the reaction is complete, add hydrochloric acid to neutralize, The phases were separated, the organic phase was cooled and crystallized, washed with water, filtered and dried to obtain 102 g of a white solid with a purity of ≥98% by weight and a particle size of D90=188.60 μm. Poor caking resistance.
preparation example 2
[0027] Preparation example 2: 120 grams of 1-(4-chlorophenethyl)-1-tert-butyl-1,2-oxirane, 6 grams of potassium hydroxide, 40 grams of 1,2,4,-triazole, Add 1 g of N,N-dimethylaniline and 100 ml of dimethylformamide into a 500 ml three-necked flask with a stirring and reflux tube, and stir for 4 hours at a reflux temperature of 130 ° C. After the reaction is complete, add hydrochloric acid for neutralization and negative pressure The solvent was recovered by distillation, the organic phase was cooled and crystallized, washed with water, filtered and dried to obtain 106 g of a white solid with a purity of ≥98.6% by weight and a particle size of D90=180.85 μm. Poor caking resistance.
preparation example 3
[0028] Preparation Example 3: 120 grams of 1-(4-chlorophenethyl)-1-tert-butyl-1,2-oxirane, 7 grams of sodium ethoxide, 40 grams of 1,2,4,-triazole, 0.5 Add 1 g of N,N-dimethyl-4-aminopyridine and 100 ml of n-butanol into a 500 ml three-neck flask with a stirring and reflux tube, and stir for 6 hours at a reflux temperature of 120 ° C. After the reaction is complete, add hydrochloric acid to neutralize and negatively The solvent was recovered by distillation under pressure, the organic phase was cooled and crystallized, filtered and dried to obtain 101 grams of white solid, the purity was ≥98.3% by weight, and the particle size D90=192.47 microns. Poor caking resistance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com