Industrial production method for preparing 2-chloro-5-picoline
A picoline and production method technology, which is applied in the production field of pesticide intermediates, can solve the problems of low cost and achieve the effects of good product quality, less tar, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
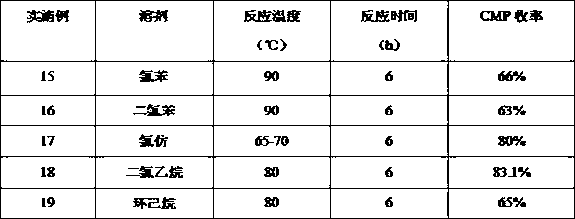
Image
Examples
Embodiment 1
[0021] Weigh 27.75g of 5-methyl-3,4-dihydro-2(1H)pyridone, and prepare 5-methyl-3,4-dihydro-2( 1H) A solution of pyridone in dichloroethane.
[0022] Weigh 61.9 g of solid phosgene and dissolve it in dichloroethane to prepare a phosgene dichloroethane solution with a mass fraction of 34.8%.
[0023] Put the prepared 5-methyl-3,4-dihydro-2(1H)pyridone in dichloroethane solution in the reaction vessel, heat it, when the temperature of the solution in the reaction vessel rises to 20-30°C, pass it into Chlorine 17.8 g, stop when the solution in the container is observed to be bright yellow.
[0024] Add catalyst—N,N-dimethylformamide (DMF) 3.3 g to the above reaction solution, stir and heat up to reflux, then add phosgene dichloroethane solution dropwise, dropwise time is 2 hours, after dropwise addition, in Keep warm under reflux for 6 hours, reflux to drive off excess chlorinating agent for 0.5 hour, lower the reaction system to room temperature, then add dilute alkali dropwis...
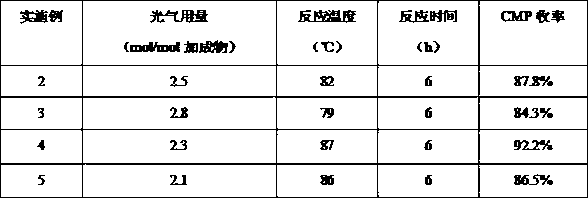
example 2-5
[0027] In order to investigate the effect of the amount of phosgene on the synthesis of 2-chloro-5-picoline (CMP), experiments were carried out in which different amounts of phosgene participated in the reaction, and the rest of the conditions were the same as in Example 1. The data is as follows:
[0028]
[0029] It can be seen from the above table that under the same reaction conditions, when the molar ratio of phosgene to adduct is 2.3, the yield of monochlorine is the highest, which is 92.2%. Too much or too little amount of phosgene will lead to a decrease in the yield of monochlorine.
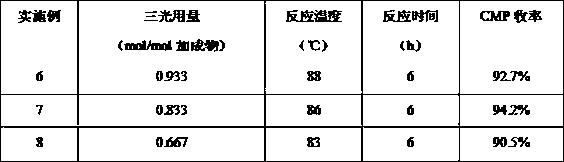
example 6-8
[0031] In above-mentioned example 1, replace the phosgene dichloroethane solution with triphotodichloroethane liquid, all the other remain unchanged. The following are the experimental results under different Sanguang dosages.
[0032]
[0033] It can be seen from the above table that under the same reaction conditions, when the molar ratio of Sanguang to the adduct is 0.833, the yield of monochlorine is the highest, which is 94.2%. However, the dosage of Sanguang is too high or the high and low yields are all reduced.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap