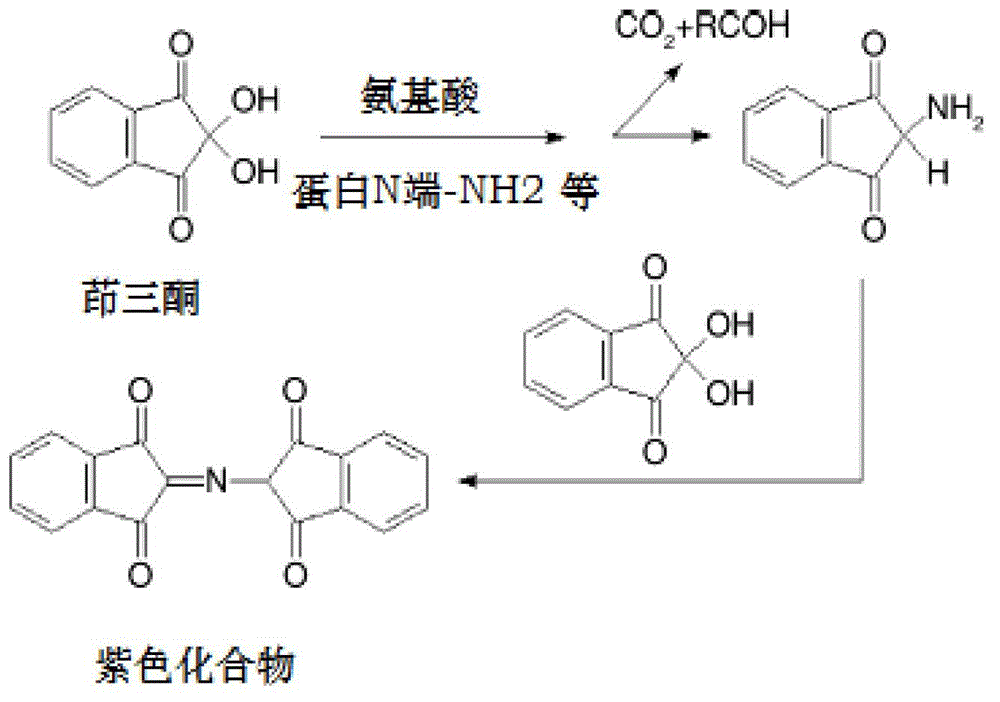
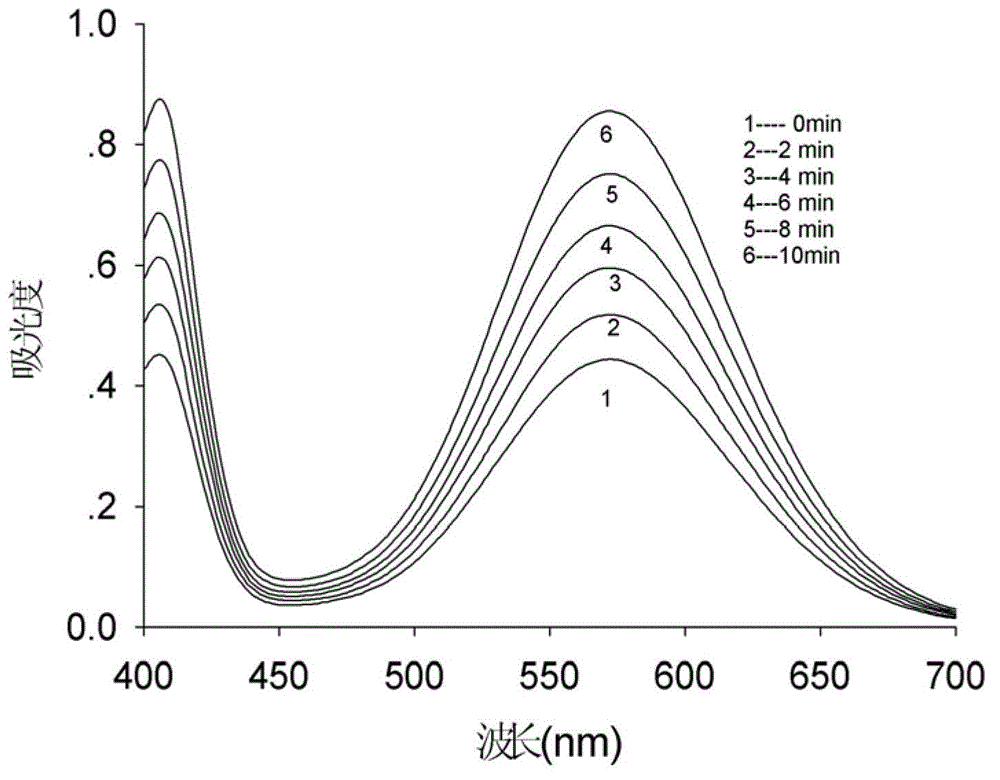
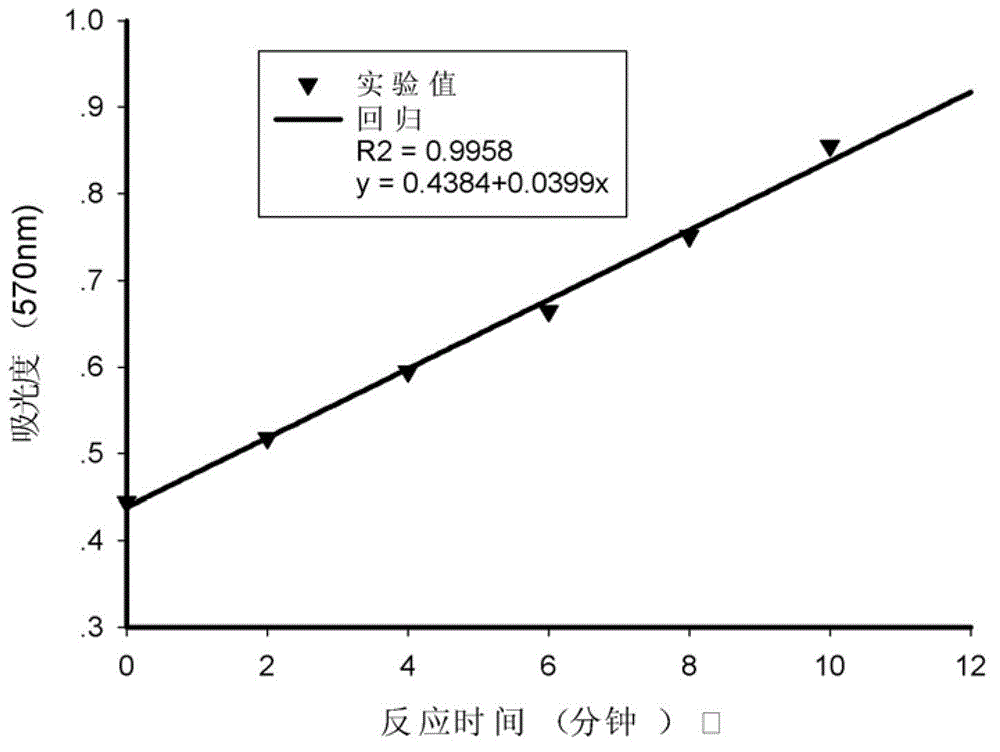
Method for screening protease inhibitor
A technology for protease inhibitors and screening methods, applied in the field of screening protease inhibitors, can solve the problems of artificial synthesis of substrates, unfavorable reactions, time-consuming and labor-intensive, etc., and achieves the effects of small impact, shortened operation time, and improved repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 solution (1)
[0068] 1. Ninhydrin reaction solution
[0069] Solution A: stannous chloride-sodium citrate solution, containing 0.2M sodium citrate, 7mM stannous chloride, pH 5.0.
[0070] Solution B: ninhydrin-dimethyl sulfoxide solution, comprising dissolving ninhydrin in DMSO solution so that the final concentration of ninhydrin is 50.0 g / L.
[0071] Mix solution A and solution B in equal volumes to obtain a ninhydrin reaction solution.
[0072] 2. Reaction buffer
[0073] 50mM Tris-HCl, 150mM NaCl, 3mM CaCl 2 , 1 μM ZnCl 2 ,pH=7.5
[0074] 3. The reaction termination solution is
[0075] 50mM EDTA-Tris-HCl buffer containing 12% polyethylene glycol 6000.
Embodiment 2
[0076] The preparation of embodiment 2 solution (2)
[0077] 1. Ninhydrin reaction solution
[0078] Solution A: stannous chloride-sodium citrate solution, containing 0.1M sodium citrate, 8mM stannous chloride, pH 5.0.
[0079] Solution B: ninhydrin-DMF solution, which is dissolving ninhydrin in N,N-dimethylformamide (DMF) solution, so that the final concentration of ninhydrin is 40.0 g / L.
[0080] Mix solution A and solution B in equal volumes to obtain a ninhydrin reaction solution.
[0081] 2. Reaction buffer
[0082] 50mM Tris-HCl, 100mM NaCl, 1mM CaCl 2 , 1 μM ZnCl 2 ,pH=7.5
[0083] 3. Reaction termination solution
[0084] 25mM EDTA-Tris-HCl buffer containing 18% polyethylene glycol 4000.
Embodiment 3
[0085] The preparation of embodiment 3 solution (3)
[0086] 1. Ninhydrin reaction solution
[0087] Solution A: stannous chloride-sodium citrate solution, containing 0.2M sodium citrate, 8mM stannous chloride, pH 5.0.
[0088] Solution B: ninhydrin-ethylene glycol solution, which is dissolving ninhydrin in ethylene glycol solution so that the final concentration of ninhydrin is 60.0 g / L.
[0089] Mix solution A and solution B in equal volumes to obtain a ninhydrin reaction solution.
[0090] 2. Reaction buffer
[0091] 50mM Tris-HCl, 250mM NaCl, 5mM CaCl 2 , 1 μM ZnCl 2 ,pH=7.5,
[0092] Contains 3% polyethylene glycol 6000
[0093] 3. The reaction termination solution is 100 mM EDTA-Tris-HCl buffer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com