Systems and methods for providing a closed venting hazardous drug IV set
A delivery system, dangerous technology, applied in the direction of drug devices, filter accessories, devices introduced into the body, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Preferred embodiments of the present invention will be better understood with reference to the accompanying drawings, wherein like reference numerals refer to identical or functionally similar elements. It will be readily understood that the components of the invention as generally described and illustrated in the accompanying drawings can be arranged and designed in a number of different configurations. Accordingly, the following more detailed description (as shown in the figures) is not intended to limit the scope of the claimed invention, but merely represents preferred embodiments of the invention.
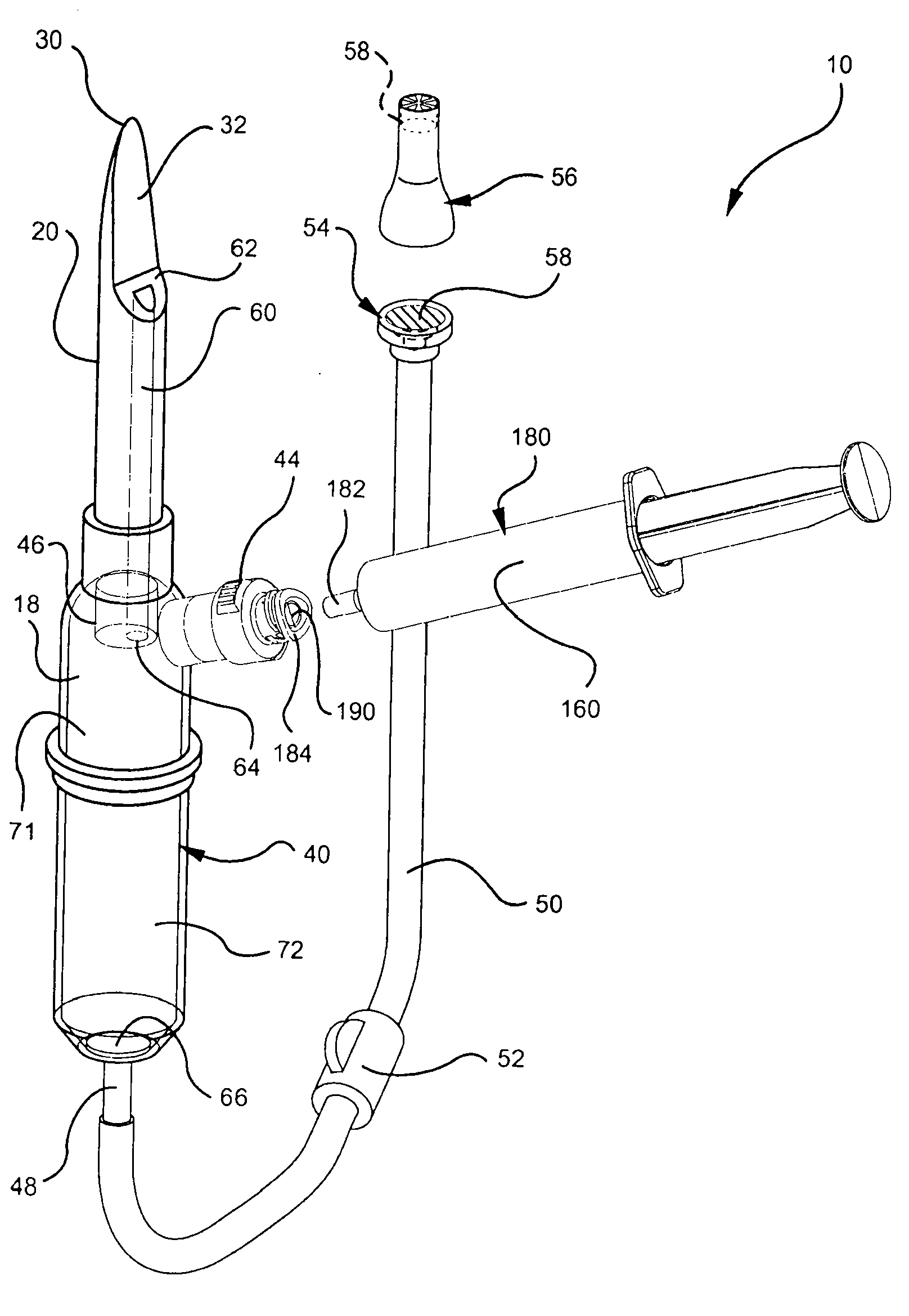
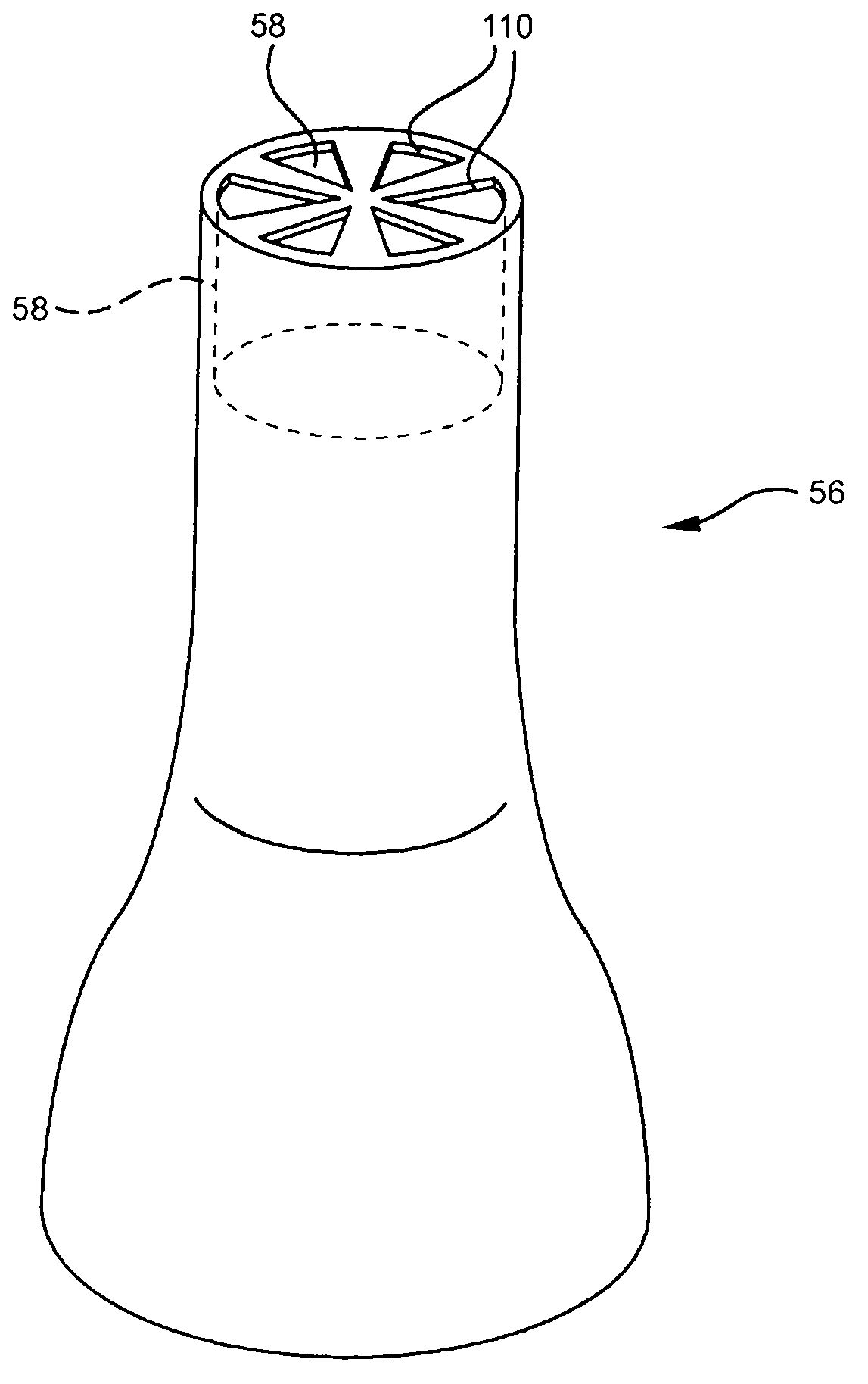
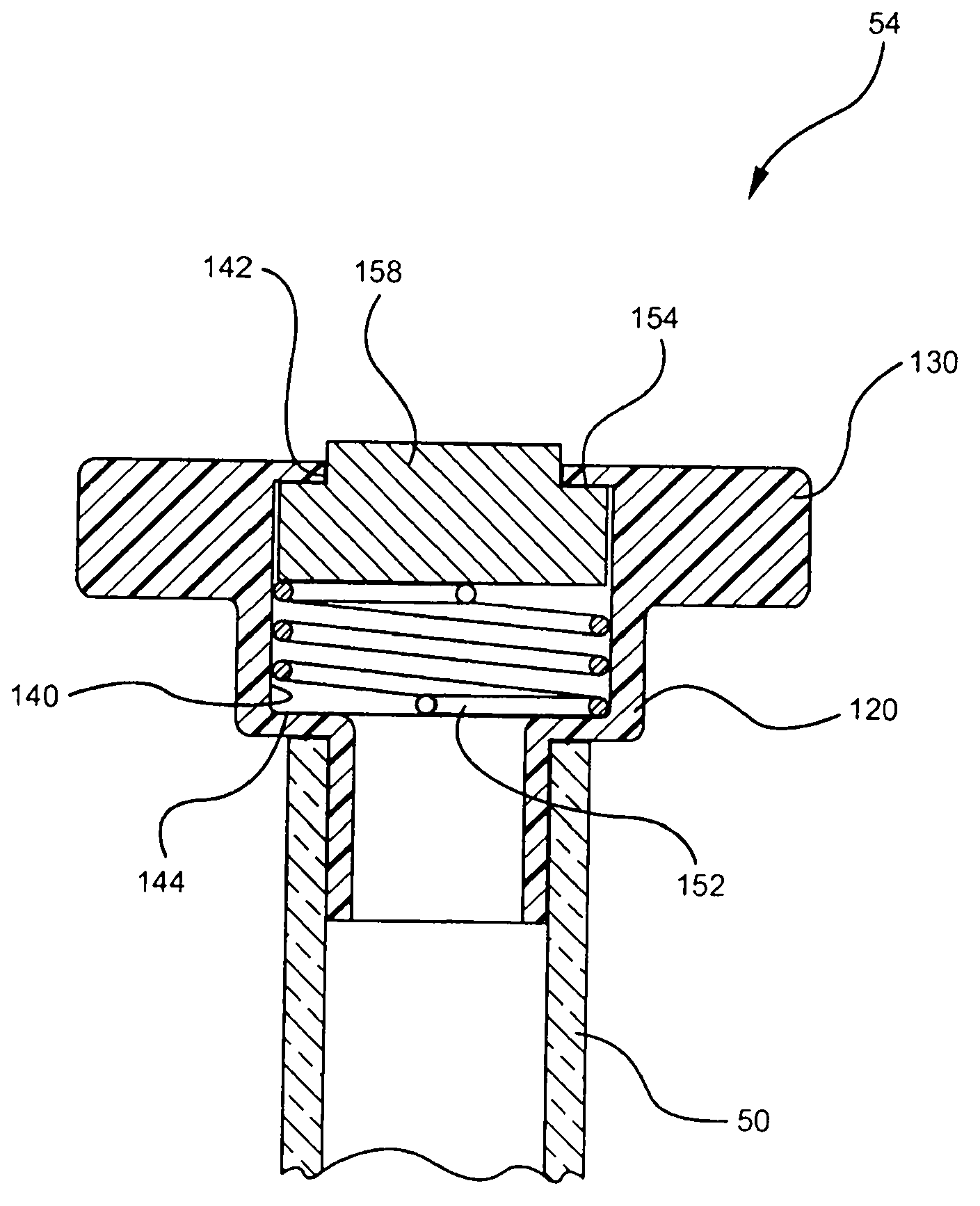
[0029] Referring now to FIG. 1 , an embodiment of an intravenous (IV) delivery system 10 is shown. Certain embodiments of the intravenous delivery system 10 include a coupling assembly 20 having a trocar 30 configured for insertion into the fluid reservoir 12, such as image 3 shown in. Certain embodiments of the coupling assembly 20 include rigid polymeric materials ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com