Monoclonal antibody against pbp2a derived from mrsa with dual binding activities
A monoclonal antibody, binding activity technology, applied in antibacterial immunoglobulins, instruments, depsipeptides, etc., can solve problems such as low affinity and drug resistance
Inactive Publication Date: 2013-01-23
RAYBIOTECH INC GUANGZHOU
View PDF2 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This bacterium is widely resistant to a variety of commonly used antibiotics, including methicillin, and its main drug resistance mechanism is due to the penicillin binding protein 2a (Penicillin Binding Protein 2a, PBP 2a) produced by MRSA bacteria, with a protein molecular weight of 75kDa; The protein is encoded by the bacterial chromosome, i.e. the mecA gene. A large number of experiments have confirmed that PBP2a exhibits low-affinity binding to β-lactam antibiotics, leading to drug resistance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
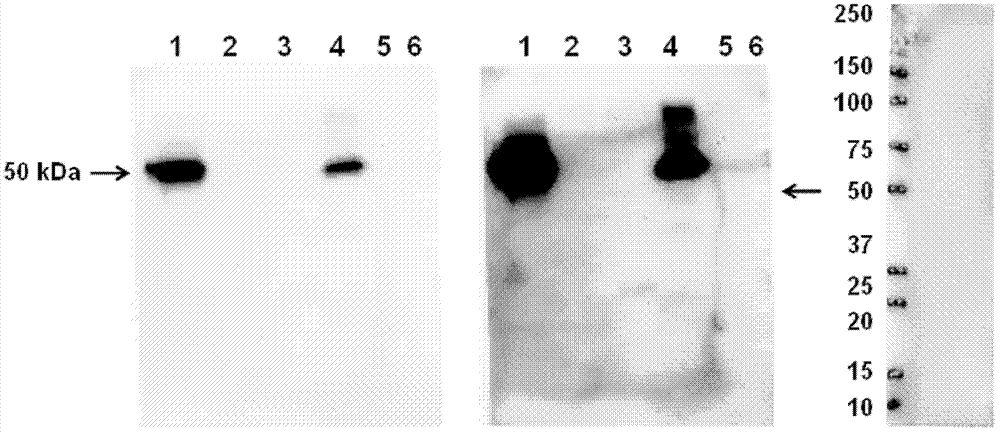
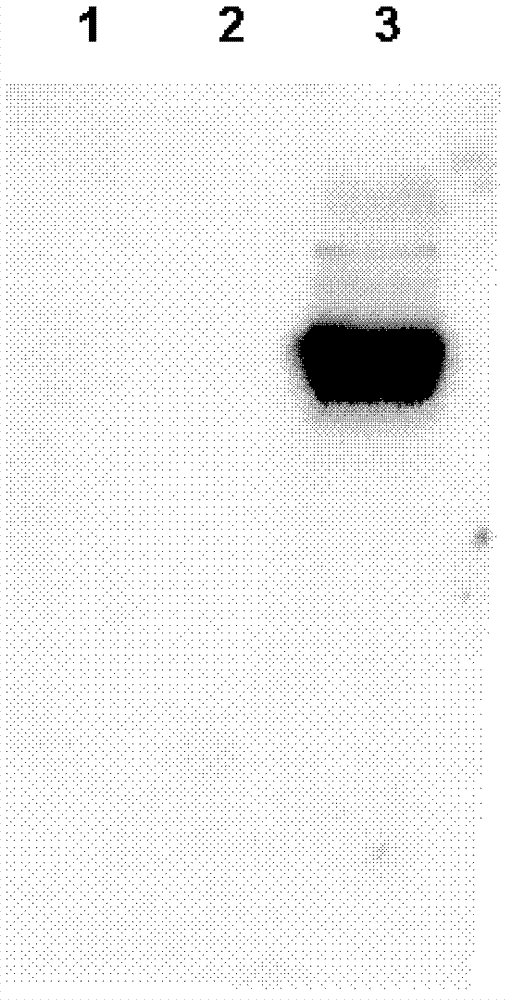
[0062] Figure 1. The primary antibody used in western blotting is purified mouse anti-MRSA antibody (2 μg / ml), the secondary antibody is HRP-labeled anti-mouse secondary antibody (1:5000), and then the specific protein bound to the antibody is detected by a chemiluminescent system. The arrows in the figure indicate that the antibody specifically recognizes and binds to the target protein in the bacterial sample.
[0063]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
Mouse monoclonal antibodies specifically recognizing the Penicillin Binding Protein 2a (PBP2a) derived from a strain of Methicillin-Resistant Staphylococcus aureus (MRSA) were produced and characterized. The immunogen used to generate an immune response in a mouse was a PBP2a recombinant protein derived from a strain of Methicillin-Resistant Staphylococcus aureus (MRSA). The data showed that both monoclonal antibodies of the disclosure were able to distinguish MRSA from MSSA bacteria. The monoclonal antibodies have distinct recognition patterns for the regions of the PBP2a protein sequence. Epitope mapping has localized regions of the PBP2a protein specifically recognized by one or both of the monoclonal antibodies. The monoclonal antibodies of the present disclosure having the ability to distinguish between MRSA and MSSA strains can be useful as the basis for a diagnostic assay useful in the clinical setting for determining whether and which antibiotics to administer to a patient.
Description
Technical field: [0001] The invention belongs to the technical field of monoclonal antibodies (technical field of biomedicine), in particular, a monoclonal antibody capable of specifically recognizing epitopes of Staphylococcus aureus (MRSA) and capable of distinguishing MRSA and MSSA has been confirmed by experiments. Background technique: [0002] MRSA (methicillin-resistant Staphylococcus aureus), that is, methicillin-resistant Staphylococcus aureus, is one of the most important drug-resistant pathogens causing infection in patients worldwide. This bacterium is widely resistant to a variety of commonly used antibiotics, including methicillin, and its main drug resistance mechanism is due to the penicillin binding protein 2a (Penicillin Binding Protein 2a, PBP 2a) produced by MRSA bacteria, with a protein molecular weight of 75kDa; The protein is encoded by the bacterial chromosome, that is, the mecA gene. A large number of experiments have confirmed that PBP2a exhibits lo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K16/12C07K7/08C07K14/31G01N33/577
CPCC07K7/08G01N33/577C07K2317/33C07K16/1271C07K16/12G01N33/56938C07K14/31
Inventor 黄若磐张英
Owner RAYBIOTECH INC GUANGZHOU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com