Novel in vitro model of human placental barrier
A human placenta and barrier body technology, applied to embryonic cells, vertebrate cells, animal cells, etc., can solve problems such as umbilical vessel stenosis, easily damaged tissues, and placental permeability that cannot be accurately evaluated by drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] 1. Preparation of reagents
[0034] 1. KRBB: Weigh NaCl 6.923g, KCl 0.354g, MgSO 4 ·7H 2 O 0.288g, CaCl 2 ·6H 2 O 0.56g, NaHCO 3 2.1g, Na 2 HPO 4 Mix 0.136g, glucose 1.8g, L-alanine 0.178g, fully dissolve with 800mL double distilled water, adjust the pH value to 7.4, make up the volume to 1000mL with double distilled water, pass through a 0.22μm filter membrane, aliquot, 4℃ save.
[0035] 2. Cryoprotectant solution A: Fully mix 7.5% ethylene glycol, 7.5% DMSO, and 85% pure water to prepare a solution.
[0036] 3. Cryoprotectant solution B: fully mix 15% ethylene glycol, 15% DMSO, and 70% pure water to make a solution.
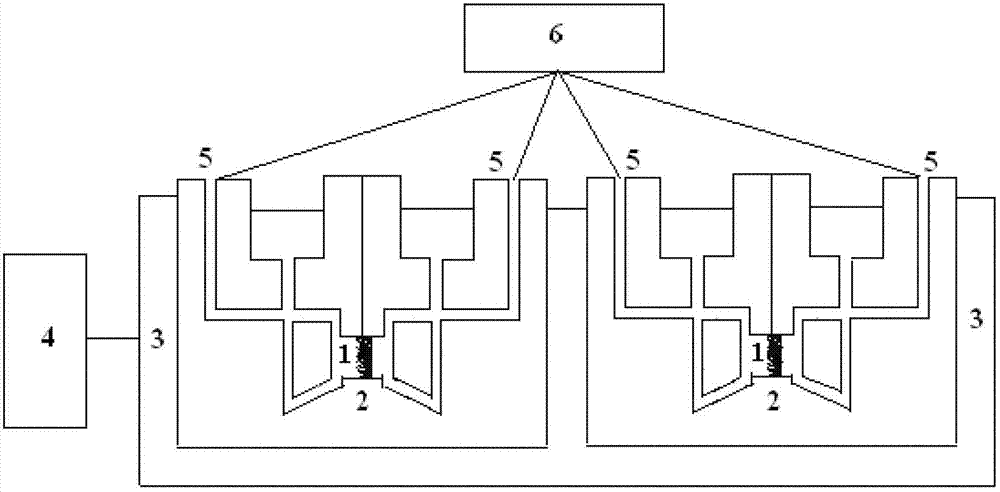
[0037] 2. Establishment of human placental barrier in vitro model
[0038] 1. Preparation of Placental Tissue Thin Sections
[0039] 1) Collect the placenta: Select the placenta of healthy women who are 25-30 years old and have a full-term delivery at 36-40 weeks of gestation. After the placenta is delivered, wash the placenta three times wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com