Aromatic hyperbranched polymer surfactant and preparation method thereof
A technology of hyperbranched polymers and surfactants, applied in chemical instruments and methods, chemical/physical processes, transportation and packaging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
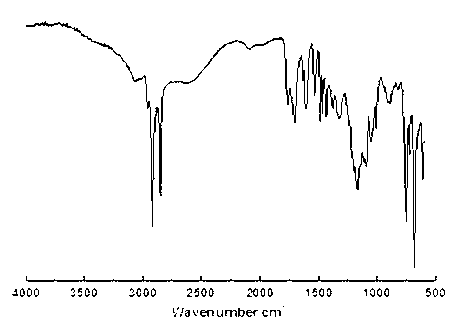
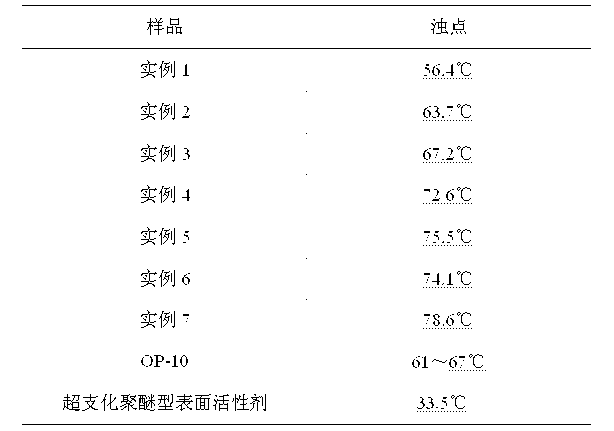
[0017] Example 1: Mix 0.1 mol of trimethylolpropane with 0.3 mol of gallic acid, add pyridine to dissolve, add 3% p-toluenesulfonic acid of the total reactant mass, stir at 120°C for 4 hours, and remove under reduced pressure at 10KPa Pyridine yields hydroxyl-terminated hyperbranched polymers. Mix the hydroxyl-terminated hyperbranched polymer and lauric acid, wherein the molar ratio of the hydroxyl group of the hydroxyl-terminated hyperbranched polymer to the lauric acid carboxyl group is 1:2, add pyridine to dissolve the reactant, and then add 3% p-toluene of the total reactant mass The sulfonic acid was reacted at 120°C for 6 hours to obtain the crude product. The obtained crude product was dissolved in 20 mL of chloroform, and the solution was added dropwise to 50 mL of deionized water for precipitation. The lower layer solution was taken and the solvent chloroform was removed under reduced pressure at 10 KPa. The obtained pale yellow solid was an aromatic hyperbranched sur...
Embodiment 2
[0019] Example 2: Mix 0.1mol of trimethylolethane with 0.6mol of gallic acid, add pyridine to dissolve, add 5% concentrated sulfuric acid of total reactant mass, stir at 110°C for 3 hours, remove pyridine under reduced pressure at 30KPa A hydroxyl-terminated hyperbranched polymer is obtained. Mix the hydroxyl-terminated hyperbranched polymer and palmitic acid, wherein the molar ratio of the hydroxyl group of the hydroxyl-terminated hyperbranched polymer to the palmitic acid carboxyl group is 1:1, add pyridine to dissolve the reactant, and then add 5% concentrated sulfuric acid of the total reactant mass , and reacted at 110 °C for 4 hours to obtain the crude product. The obtained crude product was dissolved in 50 mL of chloroform, and the solution was added dropwise to 200 mL of deionized water for precipitation. The lower layer solution was taken and the solvent chloroform was removed under reduced pressure at 12 KPa. The obtained pale yellow solid was an aromatic hyperbranch...
Embodiment 3
[0020] Example 3: Mix 0.1 mol of pentaerythritol with 0.4 mol of gallic acid, add pyridine to dissolve, add 5% p-toluenesulfonic acid of the total reactant mass, stir at 130° C. for 3 hours, remove pyridine under reduced pressure at 18KPa to obtain terminal hydroxyl groups Hyperbranched polymers. Mix the hydroxyl-terminated hyperbranched polymer and octanoic acid, wherein the molar ratio of the hydroxyl group of the hydroxyl-terminated hyperbranched polymer to the carboxyl group of octanoic acid is 1:2, add pyridine to dissolve the reactant, and then add 5% p-toluenesulfonic acid of the total reactant mass , and reacted at 120 °C for 4 hours to obtain the crude product. The obtained crude product was dissolved in 30 mL of chloroform, and the solution was added dropwise to 100 mL of deionized water for precipitation. The lower layer solution was taken out and the solvent chloroform was removed under reduced pressure at 15 KPa. The obtained pale yellow solid was an aromatic hype...
PUM
| Property | Measurement | Unit |
|---|---|---|
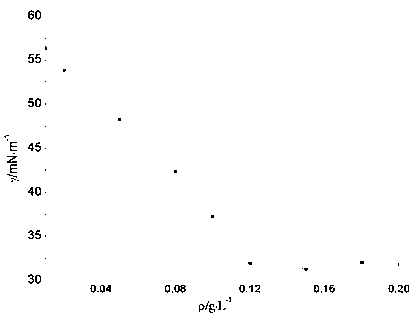
| critical micelle concentration (mass) | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com