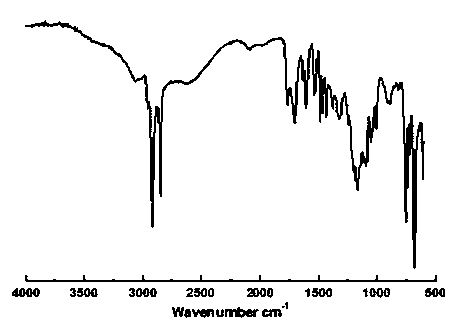
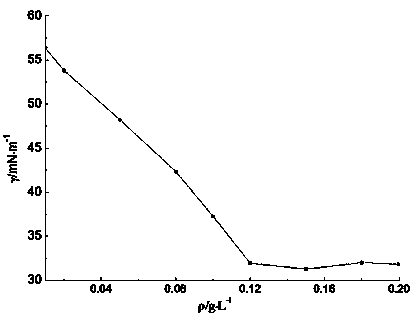
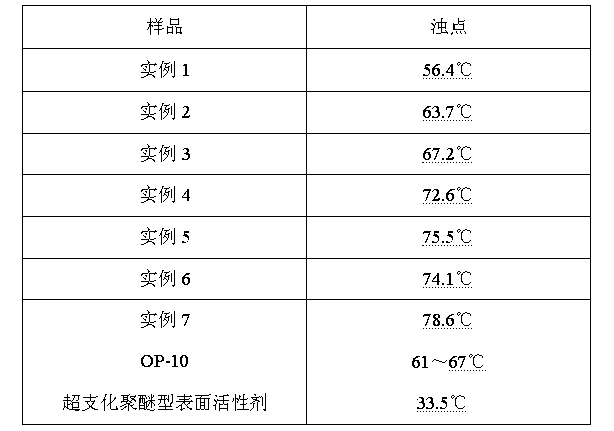
Aromatic hyperbranched polymer surfactant and preparation method thereof
一种超支化聚合物、表面活性剂的技术,应用在化学仪器和方法、化学/物理过程、运输和包装等方向,能够解决高浊点、浊点低等问题,达到好润湿性能、好乳化分散作用、好自我稳定性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Mix 0.1mol trimethylolpropane with 0.3mol gallic acid, add pyridine to dissolve, add p-toluenesulfonic acid with 3% total reactant mass, stir at 120°C for 4 hours, remove under reduced pressure at 10KPa Pyridine yields hydroxyl-terminated hyperbranched polymers. Mix the hydroxyl-terminated hyperbranched polymer and lauric acid, wherein the molar ratio of the hydroxyl group of the hydroxyl-terminated hyperbranched polymer to the carboxyl group of lauric acid is 1:2, add pyridine to dissolve the reactant, and then add 3% p-toluene of the total reactant mass Sulfonic acid was reacted at 120°C for 6 hours to obtain a crude product. The obtained crude product was dissolved in 20 mL of chloroform, and then the solution was added dropwise to 50 mL of deionized water for precipitation, and the lower layer solution was removed under reduced pressure at 10 KPa to remove the solvent chloroform, and the obtained pale yellow solid was an aromatic hyperbranched surfactant....
Embodiment 2
[0019] Example 2: Mix 0.1mol trimethylolethane with 0.6mol gallic acid, add pyridine to dissolve, add concentrated sulfuric acid with 5% total reactant mass, stir for 3 hours at 110°C, remove pyridine under reduced pressure at 30KPa A hydroxyl-terminated hyperbranched polymer is obtained. Mix the hydroxyl-terminated hyperbranched polymer with palmitic acid, wherein the molar ratio of the hydroxyl-terminated hyperbranched polymer to the palmitic acid carboxyl group is 1:1, add pyridine to dissolve the reactant, and then add concentrated sulfuric acid with 5% of the total reactant mass , reacted at 110° C. for 4 hours to obtain a crude product. The obtained crude product was dissolved in 50 mL of chloroform, and then the solution was added dropwise to 200 mL of deionized water for precipitation, and the lower layer solution was removed under reduced pressure at 12 KPa to remove the solvent chloroform, and the obtained pale yellow solid was an aromatic hyperbranched surfactant. ...
Embodiment 3
[0020] Example 3: Mix 0.1 mol of pentaerythritol with 0.4 mol of gallic acid, add pyridine to dissolve, add 5% p-toluenesulfonic acid of the total reactant mass, stir at 130°C for 3 hours, remove pyridine under reduced pressure at 18KPa to obtain terminal hydroxyl hyperbranched polymers. Mix the hydroxyl-terminated hyperbranched polymer and octanoic acid, wherein the molar ratio of the hydroxyl group of the hydroxyl-terminated hyperbranched polymer to the octanoic acid carboxyl group is 1:2, add pyridine to dissolve the reactant, and then add 5% p-toluenesulfonic acid of the total reactant mass , and reacted at 120° C. for 4 hours to obtain a crude product. The obtained crude product was dissolved in 30 mL of chloroform, and then the solution was added dropwise to 100 mL of deionized water for precipitation, and the lower layer solution was removed under reduced pressure at 15 KPa to remove the solvent chloroform, and the obtained light yellow solid was an aromatic hyperbranch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| cloud point | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com