Method for treating colorectal cancer
A colorectal cancer and colon technology, applied in chemical instruments and methods, pharmaceutical formulations, antibody medical components, etc., can solve the problems of high mortality risk rate and few treatment options for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
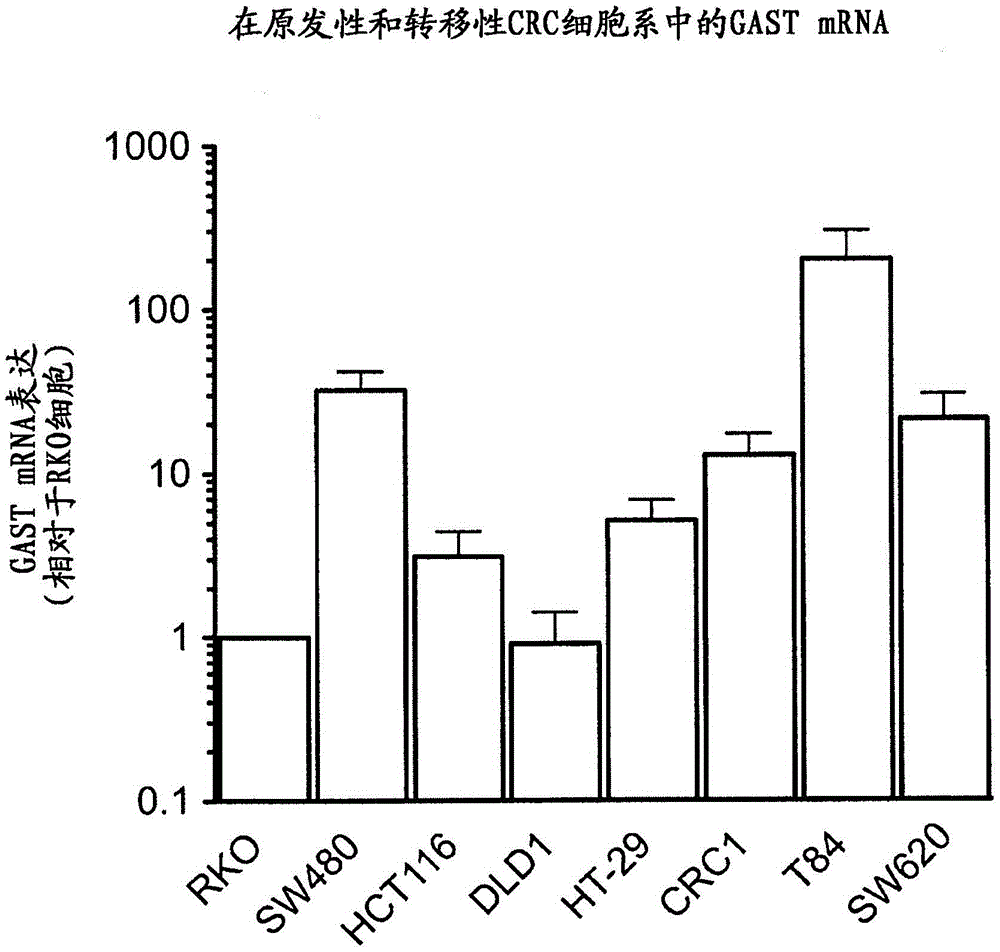
[0307] Example 1 Expression of Gastrin Gene in Metastatic Colorectal Cancer Cells
[0308] This example describes the expression of the gastrin (GAST) gene in the human primary colorectal cancer cell lines HT29, HCT116, RKO, SW480 and DLD1 and the metastatic colorectal cancer cell lines SW620 and T84. Cells (CRC1 ) isolated from biopsies from human primary colorectal tumors were also tested. SW620 cells were originally derived from lymph node metastases of a patient diagnosed with Dukes' stage C colorectal adenocarcinoma. T84 cells were originally derived from lung metastases of patients diagnosed with colorectal cancer.
[0309] A. method
[0310] GAST mRNA expression was quantified from RNA preparations of HT29, HCT116, RKO, SW480, DLD1, SW620 and T84 cell lines using quantitative RT-PCR using standard techniques. Data are presented in comparison to gastrin mRNA expression levels present in RKO primary colorectal cancer cell lines. RKO cells normally express low levels o...
Embodiment 2
[0314] Example 2 Expression of the gastrin gene in primary and metastatic colorectal tumors surgically removed from patients
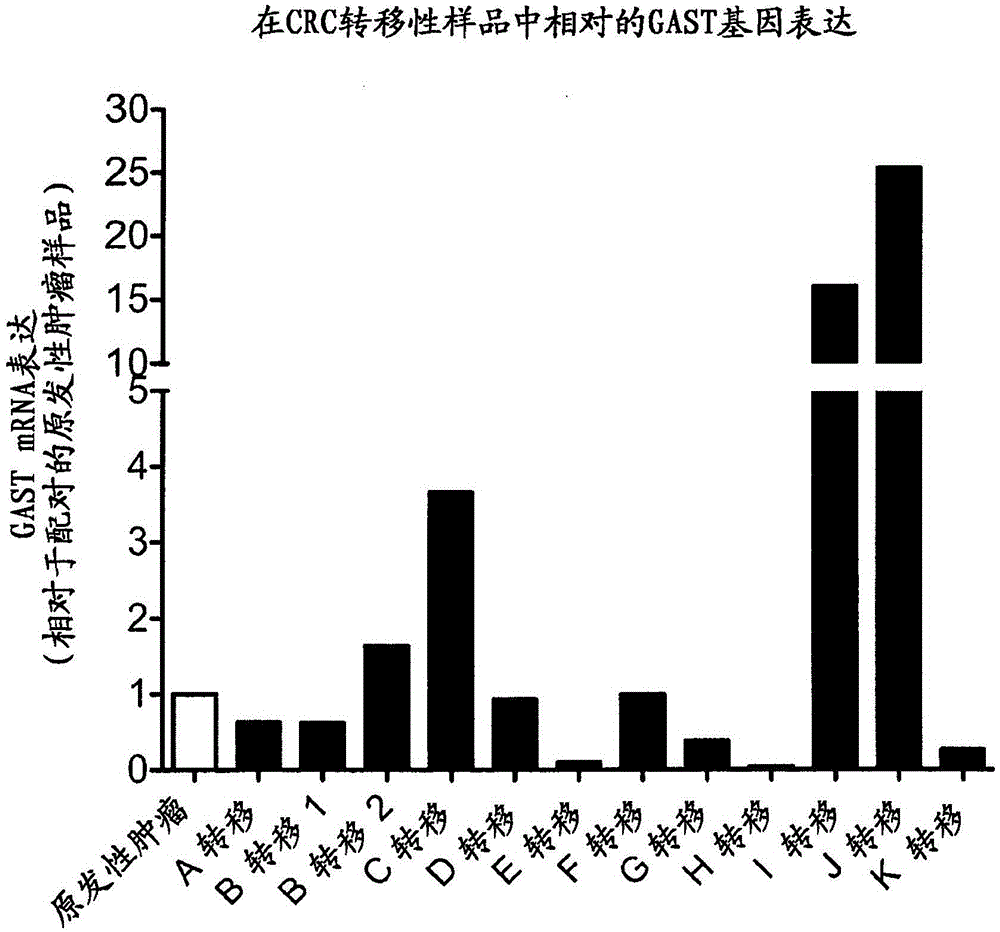
[0315] This example describes the expression of the gastrin gene (GAST) in matched primary and metastatic colorectal tumors surgically removed from patients.
[0316] A. method
[0317] Primary and metastatic colorectal tumors were surgically resected from patients according to applicable ethical guidelines. RNA was prepared from tumor tissue samples and gastrin mRNA was measured by quantitative RT-PCR using standard techniques. Gastrin mRNA expression in metastatic tumors was normalized to expression levels in matched primary tumors taken from the same patient.
[0318] B. Results
[0319] figure 2 shows the levels of gastrin mRNA expressed in metastatic colorectal tumors from 11 patients relative to expression in matched primary tumors from the same patients. Although all primary and metastatic colorectal tumors studied expressed the gastrin ge...
Embodiment 3
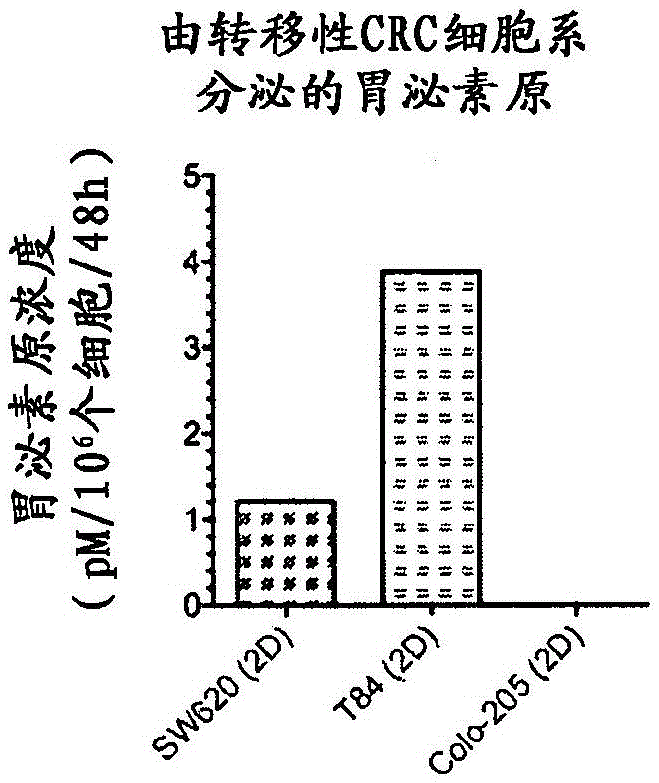
[0320] Example 3 Secretion of Progastrin by Metastatic Cancer Cells
[0321] This example describes the quantification of progastrin secretion from 3 different metastatic colorectal cancer cells.
[0322] A. method
[0323] Cells in regular 75cm 2 Grow in plastic bottles until reaching 60% confluency. The medium was then removed and the cells were rinsed once with PBS. 20 ml of M11 medium (without phenol red) was then added to each bottle, and the cells were incubated for an additional 48 hours. The medium was then harvested, centrifuged at 1,000 g for 5 minutes to remove cell debris, and frozen at -80°C. Cells were then trypsinized and counted.
[0324] To measure secreted progastrin, frozen growth medium was slowly thawed on ice and then concentrated 40-fold to a volume of 500 μl by centrifugation at 2,500 g for 45 minutes using a protein concentrator (Icon Pierce). Progastrin concentrations were subsequently measured using a sandwich ELISA technique.
[0325] B. Resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com