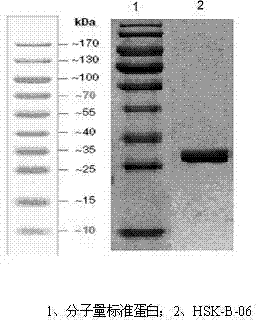
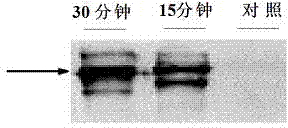
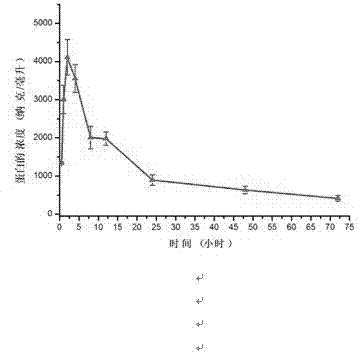
Medicine fusion specifically bound with human angiopoietin-2
An angiogenin and fusion technology, applied in the field of genetic engineering, can solve the problems of small molecular weight of small peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] This example describes the preparation of the X fragment sequence as:
[0082] QEECEWDPWTCEHM (SEQ ID NO: 1),
[0083] The sequence of X' fragments is:
[0084] MHECTWPDWECEEQ (SEQ ID NO:3)
[0085] Y is GGGKGGG (SEQ ID NO: 2)
[0086] Z is a monomethoxypolyethylene glycol with a molecular weight of 20,000 Daltons and activated by maleic imide at the end
[0087] a and b do not exist, that is, the number of amino acid residues is 0
[0088] n=2
[0089] The preparation method of the drug fusion body, the fusion body is named HSK-B-06 in the present invention.
[0090] HSK-B-06 plasmid clone
[0091] Cloning of plasmids expressing the following protein sequences:
[0092] QEECEWDPWTCEHMQEECEWDPWTCEHMGGGKGGGMHECTWPDWECEEQ MHECTWPDWECEEQ (SEQ ID NO: 4)
[0093] Primer 5' CATGCCATGGCC CAT ATG AAA TAC-3' (SEQ ID NO: 5)
[0094] 5'-CCGAATTCTCATTACTGTTCTTCGC-3' (SEQ ID NO:6)
[0095] The DNA sequence encoding the above protein sequence is as follows:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com