Application of tanshinone IIA or pharmaceutically acceptable salt thereof in improving exercise tolerance of pulmonary vascular disease patients
A vascular disease, tanshinone technology, applied in the new application field of tanshinone IIA, can solve the problems of limited activity, decreased activity tolerance, limited curative effect, etc., and achieves the effect of low price and improved activity tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
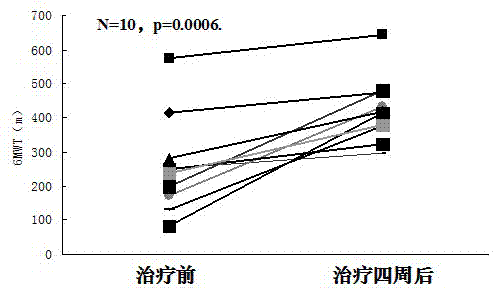
[0026] Example 1: Tanshinone IIA can significantly increase the average six-minute walking distance in patients with pulmonary arterial hypertension after four weeks of treatment
[0027] 1. Patient Information
[0028] There were 10 patients, including 4 males and 6 females, aged 17-62 years.
[0029] 2. Inclusion criteria
[0030] All patients enrolled in the group were diagnosed as patients with pulmonary arterial hypertension according to the "ESC2009 Guidelines for the Diagnosis, Treatment and Treatment of Pulmonary Hypertension".
[0031] 3. Experimental plan
[0032] After the patients were enrolled in the group, they performed a six-minute walk test (6MWT), recorded the average six-minute walk distance before the experiment, and then received conventional treatment according to their condition, and gave Tanshinone IIA Sodium Sulfonate Injection 1mg / Kg·body weight, plus 5% glucose injection Liquid, intravenous infusion, once a day, the treatment cycle is 4 we...
Embodiment 2
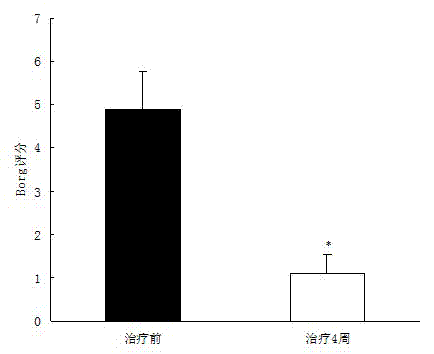
[0035] Example 2: Tanshinone IIA can significantly improve dyspnea and fatigue scores in patients with pulmonary arterial hypertension after four weeks of treatment
[0036] 1. Patient Information
[0037] There were 10 patients, including 4 males and 6 females, aged 17-62 years.
[0038] 2. Inclusion criteria
[0039] All patients enrolled in the group were diagnosed as patients with pulmonary arterial hypertension according to the "ESC2009 Guidelines for the Diagnosis, Treatment and Treatment of Pulmonary Hypertension".
[0040] 3. Experimental plan
[0041] After the patients were enrolled in the group, the patients were asked to use the Borg Dyspnea and Fatigue Scale to score their dyspnea and fatigue (Borg score), record the score before the experiment, and then receive routine treatment according to their condition, and give Tanshinone IIA Sodium Sulfonate Injection 1mg / Kg · body weight, plus 5% glucose injection, intravenous infusion, once a day, the treatme...
Embodiment 3
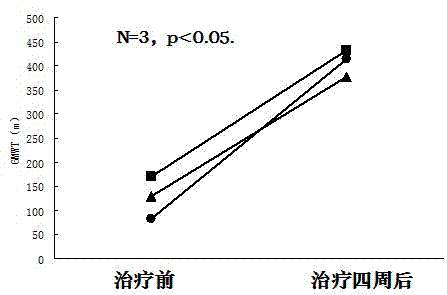
[0044] Example 3: Tanshinone IIA can significantly increase the average six-minute walking distance in patients with chronic thromboembolic pulmonary hypertension after four weeks of treatment
[0045] 1. Patient Information
[0046] There were 3 patients, all female, aged 46-62 years.
[0047] 2. Inclusion criteria
[0048] All patients enrolled in the group were diagnosed as patients with chronic thromboembolic pulmonary hypertension according to the "ESC2009 Guidelines for the Diagnosis, Treatment and Treatment of Pulmonary Hypertension".
[0049] 3. Experimental plan
[0050] After the patients were enrolled in the group, they performed a six-minute walk test (6MWT), recorded the average six-minute walk distance before the experiment, and then received conventional treatment according to their condition, and gave Tanshinone IIA Sodium Sulfonate Injection 1mg / Kg·body weight, plus 5% glucose injection Liquid, intravenous infusion, once a day, the treatment cycle i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com